Taking Nature's Pulse: Section 2: B.C.'s Natural Legacy
2. British Columbia's Natural Legacy
To describe the beauties of this region will, on some future occasion be a very grateful task to the pen of a skilled panegyrist.a The serenity of the climate, the innumerable pleasing landscapes, and the abundant fertility that unassisted nature puts forth [renders] it the most lovely country that can be imagined.
- Captain George Vancouver, 1792.126
Prior to Europeans arriving at the end of the 18th century on the shores of the land we now call British Columbia, the population of First Peoples is estimated to have been between 80,000 and 250,000.127 Clues to the abundance and diversity that existed here at that time can be found in First Nations stories and legends and in the journals of early European visitors who regarded the landscapes and wildlife as remarkable. Historical ecosystem mapping shows that Garry oak ecosystems were common along the southeast coast of Vancouver Island and extensive grasslands flourished throughout the Okanagan, Thompson and Nicola valleys.128,129 Robert Brown, the first colonial to cross Vancouver Island on foot, describes the deer being "so thick that you only require to go behind a bush - sound [a] hunting whistle and take iyour pick of the fattest and best" and the lakes "merry with leaping trout and salmon."130 More than 400 grizzly bear (Ursus arctos) hides were taken from the Cascade Mountains between 1846 and 1851,b,131 and in 1918, a herd of about 2,000 mountain caribou was sighted at Isaac Lake in what is now Bowron Lake Provincial Park.c,132 Peak runs of sockeye salmon in the Fraser River in the early 1900s are estimated to have exceeded 50 million fish.133
a A panegyrist is a person who writes laudatory speeches or tributes.
b Includes a portion of the Cascade Mountains in Washington State.
c This exceeds the current estimated provincial population of mountain caribou (see Text box 13, p. 85).
While 14 species, including the passenger pigeon (Ectopistes migratorius), western pond turtle (Actinemys marmorata) and viceroy butterfly (Limenitis archippus), have disappeared from the province since European contact (see Table 14, p. 58), almost all of the native species and ecosystems that were present in B.C. in 1776, when Captain Cook stepped ashore on Nootka Island, still occur here today.
Like early European explorers, a modern visitor to the province might use the word 'remarkable' to describe B.C.'s landscapes and wildlife. There are still vast forests, mountains and great rivers; ungulates and carnivores that have disappeared from other places continue to roam the province; and enormous flocks of migratory birds still stop to rest and feed on many lakes and estuaries. But over the past two centuries there have been changes in the abundance and distribution of many native species and ecosystems.
Section 2 summarizes information on the current state of B.C.'s ecosystem, species and genetic diversity relative to pre-European contact. Each of these components is examined in relation to the terrestrial and freshwater realms, as well as to the portions of those realms that overlap with the marine realm. The overlap of species and ecosystems with other jurisdictions is also considered. This section describes recent trends where available, as well as information about key elements of B.C.'s biodiversity that are essential to the functioning of particular ecosystems and special elements that are relatively unique in the world.
2.1 Approach
The assessment of biodiversity in British Columbia was based on an examination of conservation status and proportion of global range for ecosystems, species and genes. The current status and threats are reviewed for examples of key elements and special elements.134
Conservation status indicates the level of risk of extirpation (provincially) or extinction (globally) for an element of biodiversity (e.g., an ecosystem or a species) and was examined at both the provincial and global level. Proportion of global range is the proportion of the global population (for species, subspecies, ecotypes, etc.) a or range (for ecosystems) of an element of biodiversity within B.C. This is sometimes referred to as 'global responsibility' or 'stewardship responsibility.'135 The reason for considering the proportion of an element's global range in B.C. is that it indicates the opportunity that exists for the province to influence its global status (i.e., its risk of extinction). For example, the province has the potential to have a major influence on the global status of elements that are found exclusively or predominantly in B.C. (see Section 2.5.2, p. 137).
a In the majority of cases, population data were not available and range was used as a proxy.
An additional analysis of richness was applied to species and an analysis of rarity was conducted for ecosystems. Species richness is the total number of species in an area.136 Ecosystem rarity is the proportion of an area (in this case the entire province) occupied by an ecosystem.137
Due to a lack of information, the assessment of genetic diversity was restricted to those subspecies, populations and varieties that have been identified by the B.C. Conservation Data Centre as being of conservation concern.
2.2 Ecosystem Diversity in British Columbia
The province of British Columbia stretches from the 48th parallel at its most southerly point on Vancouver Island to the 60th parallel in the north, and ranges in elevation from sea level along the coast to over 4,000 m at the peaks of the highest mountains - Mount Waddington in the Coast Mountains and Mount Fairweather at the south end of the St. Elias Mountains on the Alaska-B.C. border.138 On the coast, warm, moist air from the Pacific Ocean releases its moisture as rain or snow as it rises over the mountains, producing the highest rainfall and some of the most productive forests in Canada.139 Much of the province is covered by the Cordilleran mountain system of western North America, with the Coast Mountains to the west and the Cassiar-Omineca, Cariboo, Columbia and Rocky mountains to the east. These mountain systems give rise to British Columbia's great rivers: the Fraser, Thompson, Kootenay, Columbia, Parsnip, Finlay, Peace, Kechika, Liard, Skeena, Nass, Stikine and Taku. Two high inland plateaus - the Interior and the Stikine - sit at an average of 1,000 m above sea level. On the Interior Plateau and in the surrounding low-elevation mountains, the continental air mass creates greater extremes of temperature and precipitation. The province's driest regions occur in the valleys of the southern interior, in the rain shadow to the east of the Coast Mountains. The warm Pacific air rises once again as it travels east, creating an interior wet belt to the west of, and within parts of, the Rockies. The Peace region in the northeast, an extension of the interior plains of Alberta and one of B.C.'s few lowland areas, is characterized by flat, rolling hills and a cold northern climate.
British Columbia's large size, intricate coastline and complex topography, and the resulting climates have created a wide array of diverse ecosystems. Almost three-quarters of the province is covered by forest (Figure 7). Most of the remaining area is covered by glaciers and alpine ecosystems, with grasslands, wetlands, lakes and streams collectively occupying only about 10% of the province's total area. Almost 10% of the province is covered by rock, with the majority (almost 9%) occurring in the alpine.140
Figure 7: Land cover types in B.C. as percent of total land area.
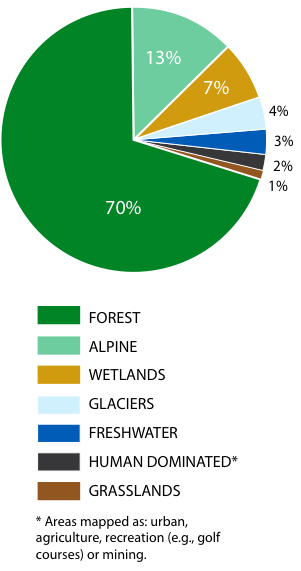
Source: Biodiversity BC, 2008. The Biodiversity Atlas of BC. Available at: www.biodiversitybc.org.
Because ecosystems are dynamic over space and time (see Section 1.1, p.5), it can be difficult to characterize an ecosystem as a discrete unit. Ecosystems can be examined at a wide range of scales, from a single rotting log in a forest to an entire forest type covering thousands of square kilometres. For the purposes of this report, terrestrial ecosystems were assessed at a broad provincial scale using a well-defined, higher-level ecosystem classification, the Biogeoclimatic Ecosystem Classification (BEC) system,141 and freshwater ecosystems were assessed at the level of Major Drainage Areas. Although there was a difference in emphasis between the two analyses, both terrestrial and freshwater systems were considered in each one.
2.2.1 Terrestrial Ecosystems: Biogeoclimatic Ecosystem Classification Zones
Biogeoclimatic Ecosystem Classification zones, commonly referred to as biogeoclimatic zones, are broad geographic areas sharing similar climate and vegetation. The BEC system was developed specifically for B.C. in the 1960s and early 1970s and continues to be revised and updated. Because biogeoclimatic zones have been well delineated, they were chosen as the broad-scale representation of ecosystems for the province. Sixteen biogeoclimatic zones are recognized for B.C. (see Map 2, p. 28). Twelve of the zones are forested, three are alpine and one is dominated by grasses (Text box 4).
Text Box 4. British Columbia's Biogeoclimatic Zones142,143,144
B.C.'s biogeoclimatic zones are each named after one or more dominant native plants, often with a geographic modifier (e.g., coastal, interior, alpine) or climatic modifier (e.g., boreal, montane).
Forested Zones
Boreal White and Black Spruce: Covers B.C.'s northeast corner and extends into valleys west of the northern Rocky Mountains at low elevations. Consists of a mix of upland forest and muskeg ecosystems, with a wide range of tree species including white spruce (Picea glauca), black spruce (P. mariana), lodgepole pine, trembling aspen (Populus tremuloides), black cottonwood (Populus balsamifera ssp. trichocarpa) and tamarack (Larix laricina).
Coastal Douglas-fir: Limited to low-elevations covering a small part of southeastern Vancouver Island, several Gulf Islands and a narrow strip of the adjacent mainland. Douglas-fir is the dominant tree, frequently accompanied by western redcedar, grand fir (Abies grandis), arbutus (Arbutus menziesii), Garry oak or red alder (Alnus rubra).
Coastal Western Hemlock: Covers most low elevations west of the Coast Mountains. Western hemlock and western redcedar are both common.
Engelmann Spruce-Subalpine Fir: Occupies the uppermost forested elevations in the southern three-quarters of the interior. Includes continuous forest dominated by Engelmann spruce and subalpine fir at lower and mid elevations, and subalpine parkland (characterized by clumps of trees scattered among areas of heath, meadow and grassland) at upper elevations.
Interior Cedar-Hemlock: Occurs in two separate parts of B.C. - the southeast and the northwest. Dominated by western redcedar and western hemlock, but has the highest diversity of tree species of any zone in the province.
Interior Douglas-fir: Occurs at low to mid elevations in the southcentral interior, including leeward slopes of the Coast Mountains and the southern Rocky Mountain Trench. Although Douglas-firdominated forests are most common, this zone features a wide array of ecosystems, including extensive grasslands in drier areas.
Montane Spruce: Occurs at mid elevations in the southern interior. Features a unique mix of species from both higher and lower zones, including hybrid spruce (Picea engelmannii x glauca; a cross between Engelmann and white spruce), subalpine fir, Douglas-fir and lodgepole pine.
Mountain Hemlock: Occupies subalpine elevations of the Coast Mountains. The most common tree species are mountain hemlock, amabilis fir and yellow-cedar, often with a dense understory of blueberries (Vaccinium spp.) and other shrubs.
Ponderosa Pine: Occurs at low elevations in very dry, southern interior valleys. Consists of a mosaic of forest and grassland, but is dominated by trees. Ponderosa pine (Pinus ponderosa) often grows in very open, park-like stands with an understory dominated by bluebunch wheatgrass (Pseudoroegneria spicata).
Spruce-Willow-Birch: Occupies subalpine elevations in the northern third of the interior. In forested ecosystems, the main tree species are white spruce and subalpine fir. Shrub-dominated ecosystems, characterized by scrub birch (Betula nana) and various willows (Salix spp.), are also common in this zone.
Sub-Boreal Pine-Spruce: Occurs on high plateaus in the central interior. Consists of two principal ecosystems: lodgepole pine forests and wetlands. Besides lodgepole pine, the only common tree species are white spruce and trembling aspen.
Sub-Boreal Spruce: Occupies the gently rolling terrain of the Interior Plateau and extends into mountainous areas to the north, west and east. Features dense coniferous forests dominated by hybrid spruce and subalpine fir.
Alpine Zones
Boreal Altai Fescue Alpine: Occurs in the northern Rocky, Skeena, Omineca and Cassiar mountains in the north and on the lee side of the Coast Mountains north of the Chilcotin. In the alpine as a whole, the primary vegetation consists of low-growing, evergreen shrubs. In this zone, the dominant species are dwarf willows, grasses (e.g., altai fescue [Festuca altaica]), sedges (Carex spp.) and lichens. Coastal Mountain-heather Alpine: Occurs along the windward spine of the Coast Mountains and the mountains of Vancouver Island and Haida Gwaii/Queen Charlotte Islands. Features extensive beds of white mountain-heather (Cassiope mertensiana var. mertensiana) and pink mountain-heather (Phyllodoce empetriformis).
Interior Mountain-heather Alpine: Occurs in the southern third of the province in the Columbia Mountains, southern Rocky Mountains and on the lee side of the Coast and Cascade mountains. Dominant vegetation ranges from mountain-heathers in snowier areas to mountain-avens (Dryas spp.) on the driest sites. grassland zone
Bunchgrass: Occupies narrow fingers of land at lower elevations along the major southern interior valleys. Dry sites are dominated by grasses, such as bluebunch wheatgrass and needle-and-thread grass (Hesperostipa comata), with a scattering of shrubs, such as big sagebrush (Artemisia tridentata), and an extensive cryptogamic crust (see Section
2.5.1.2-H, p. 111). Wetlands are also common throughout this zone.
Table 1. Areal Extent Of Biogeoclimatic Zones In B.C.
| Biogeoclimatic Zone | Area (km2) | Percentage |
| Engelmann Spruce-Subalpine Fir (ESSF) | 170,364 | 18% |
| Boreal White and Black Spruce (BWBS) | 153,367 | 17% |
| Coastal Western Hemlock (CWH) | 102,253 | 11% |
| Sub-boreal Spruce (SBS) | 92,346 | 10% |
| Spruce-Willow-Birch (SWB) | 80,101 | 9% |
| Boreal Altai Fescue Alpine (BAFA) | 76,812 | 8% |
| Coastal Mountain-heather Alpine (CMA) | 52,007 | 6% |
| Interior Cedar-Hemlock (ICH) | 50,915 | 5% |
| Interior Douglas-fir (IDF) | 40,418 | 4% |
| Mountain Hemlock (MH) | 36,572 | 4% |
| Montane Spruce (MS) | 27,795 | 3% |
| Sub-boreal Pine Spruce (SBPS) | 22,359 | 2% |
| Interior Mountain-heather Alpine (IMA) | 17,681 | 2% |
| Ponderosa Pine (PP) | 2,896 | <1% |
| Bunchgrass (BG) | 2,048 | <1% |
| Coastal Douglas-fir (CDF) | 1,310 | <1% |
| Total | 929,244 | 100% |
Source: Prepared for this report.
Notes: Areas of ecosystem conversion (see Map 12, p.161), as well as lakes and rivers, were removed from each zone for this analysis.
The zones are divided into subzones, based on differences in regional climate. Variants are still finer subdivisions of subzones, which reflect local variation within the subzone-level climate (e.g., areas that are slightly wetter or warmer than other areas in the subzone). v Table 1 summarizes the area of each of B.C.'s biogeoclimatic zones, listing them in order of rarity (from most common to rarest). The least common biogeoclimatic zones in B.C. are Coastal Douglas-fir, Bunchgrass and Ponderosa Pine, all dry, low-elevation or valley-bottom zones, which together make up less than 1% of the province's land area. The Coastal Douglas-fir zone occurs on the east coast of southern Vancouver Island and on the southern Gulf Islands and Sunshine Coast, while the Bunchgrass and Ponderosa Pine zones are found in the southern interior. The most common zones within B.C. are the Engelmann Spruce-Subalpine Fir, Boreal White and Black Spruce and Coastal Western Hemlock, all predominantly forested ecosystems.
2.2.1.1 conservation status of biogeoclimatic zones
The conservation status of each of the biogeoclimatic zones was determined using a modification of the NatureServea methods. Conservation status rankings were based on criteria that included rarity, trends and the level of threat from human activity.145 For this analysis, ecosystems ranked as Critically Imperilled (1), Imperilled (2) and Vulnerable (3) were considered to be of conservation concern in British Columbia (for rank definitions, see Table 2). Information was compiled at two scales: global (G), indicating the status of a biogeoclimatic zone in its worldwide range; and provincial or subnational (S), indicating the status of a biogeoclimatic zone within B.C.
The threat assessment completed as part of the process included the effects of:
1. Residential development (including housing and urban areas, commercial area and tourism recreation areas);
2. Agriculture and aquaculture (non-timber crops, plantations, livestock);
3. Energy production and mining (oil and gas, mining and quarrying, renewable energy);
4. Transportation and service corridors (roads and railways, utility and service lines, seismic lines, shipping lanes, flight paths);
5. Biological resource use (hunting and collecting, logging, fishing, harvesting of aquatic resources);
6. Human intrusion and disturbance (recreational and work activities);
7. Natural systems modification (fire and fire suppression, dams and water management);
8. Invasive and problem species (invasive and/or alien species, problematic native species, introduced genetic material);
9. Pollution (household, industrial, agricultural/forestry, garbage and solid waste, airborne pollution);
10. Geological events (volcanoes, earthquakes, avalanches); and
11. Climate change and severe weather (habitat shifting and alteration, droughts, temperature extremes, storms, flooding).
a NatureServe is an international network that includes the B.C. Conservation Data Centre. For more information, see www.natureserve.org/explorer .
Specific information used in the assessments included the overlap of the present and projected biogeoclimatic zone climate envelopes, (see Section 3.3.1.2, p. 186),146 the proportion of the zone with roads or other linear development features present (see Map 1, p. 2) and the proportion of the zone recently logged (see Map 19, p. 197).
Four biogeoclimatic zones are of conservation concern in the province: three in the interior (Bunchgrass, Ponderosa Pine and Interior Douglas-fir) and one on the coast (Coastal Douglas-fir) (Table 3). These zones collectively occupy less than 5% of B.C.'s area (Map 3).
Table 2. conservation status ranks for ecosystems in B.C.
| Rank | Definition | Description |
| 1 | Critically Imperilled | At very high risk of extinction or extirpation. |
| 2 | Imperilled | At high risk of extinction due to very restricted range, steep declines, or other factors. |
| 3 | Vulnerable | At moderate risk of extinction or extirpation due to a restricted range, recent and widespread declines, or other factors. |
| 4 | Apparently Secure | Uncommon but not rare, and usually widespread. Some cause for long-term concern. |
| 5 | Secure | Common or very common, and widespread. Not susceptible to extirpation or extinction under current conditions. |
| NR | Not yet Ranked | Rank is not yet assessed. |
| U | Unrankable | Suitable information is not available for ranking. |
Source: Adapted from Anions, M. 2006. Global and Provincial Status of Species in British Columbia. Biodiversity BC, Victoria, BC. 16pp. Available at: www.biodiversitybc.org.
Notes: For analyses in this report, range ranks (given when not enough information is available to score a specific rank) are rounded to the higher rank (e.g., S2S3 is rounded to S2; S2S4 is averaged to S3). See Section 2.3.2 (p. 51) for an explanation of conservation status rankings.
Boldface indicates that ecosystems with these ranks are of conservation concern.
Table 3. Conservation Status Of Biogeoclimatic Zones In B.C.
| Biogeoclimatic Zone | Conservation Status |
| Bunchgrass | Imperilled (S2) |
| Coastal Douglas-Fir | Imperilled (S2) |
| Ponderosa Pine | Imperilled/Vulnerable (S2/S3) |
| Interior Douglas-Fir | Vulnerable (S3) |
| Coastal Western Hemlock | Apparently Secure (S4) |
| Interior Cedar-Hemlock | Apparently Secure (S4) |
| Sub-Boreal Pine-Spruce | Apparently Secure (S4) |
| Boreal White And Black Spruce | Apparently Secure (S4) |
| Spruce-Willow-Birch | Apparently Secure (S4) |
| Sub-Boreal Spruce | Apparently Secure (S4) |
| Montane Spruce | Apparently Secure (S4) |
| Mountain Hemlock | Apparently Secure (S4) |
| Engelmann Spruce-Subalpine Fir | Secure (S5) |
| Coastal Mountain-Heather Alpine | Secure (S5) |
| Boreal Altai Fescue Alpine | Secure (S5) |
| Interior Mountain-Heather Alpine | Secure (S5) |
Source: Kremsater, L. 2007. Draft S Ranks and Surrogate G Ranks for BEC Zones and Draft S Ranks for Ecoprovinces and Major Drainage Areas of B.C.: Preliminary Rankings for Informing the Biodiversity Status Report and Action Plan. Biodiversity BC, Victoria, BC. 64pp. Available at: www.biodiversitybc.org.
Notes: Boldface indicates biogeoclimatic zone is of conservation concern. The global conservation status (G rank) for the biogeoclimatic zones was considered relative to the provincial conservation status (S rank) and in all cases was assumed to be similar; therefore, the G and S rankings were the same. Only the S rank is reported.
The four biogeoclimatic zones that are of conservation concern have the highest densities of species of both global and provincial conservation concern (Table 4). One hundred and forty-six species of provincial conservation concern have been recorded only in zones of conservation concern (i.e., in one or more of these zones). It is perhaps not surprising that there are higher numbers, and therefore higher densities, of species of provincial conservation concern in these rare zones, but the numbers and densities for species of global conservation concern show the same pattern, which is consistent with the assessment that these zones are also of global conservation concern.
Table 4. Distribution Of Species Of Conservation Concern In B.C. By Biogeoclimatic Zone.
| Biogeoclimatic Zone | Total Area (Km2) | Species Of Global Conservation Concern | Species Of Provincial Conservation Concern | ||
| Number of speciies | Density (# of species/1,000 km2) | Number of speciies | Density (# of species/1,000 km2) | ||
| Coastal Douglas-Fir | 1,310 | 24 | 18.3 | 170 | 129.8 |
| Bunchgrass | 2,048 | 10 | 4.9 | 165 | 80.6 |
| Ponderosa Pine | 2,896 | 10 | 3.5 | 114 | 39.4 |
| Interior Douglas-Fir | 40,418 | 27 | 0.7 | 252 | 6.2 |
| Montane Spruce | 27,795 | 12 | 0.4 | 93 | 3.3 |
| Coastal Western Hemlock | 102,253 | 40 | 0.4 | 242 | 2.4 |
| Mountain Hemlock | 36,572 | 13 | 0.4 | 45 | 1.2 |
| Interior Cedar-Hemlock | 50,915 | 17 | 0.3 | 170 | 3.3 |
| Alpine Tundra | 146,500 | 21 | 0.1 | 144 | 1.0 |
| Spruce-Willow-Birch | 80,101 | 10 | 0.1 | 68 | 0.8 |
| Engelmann Spruce-Subalpine Fir | 170,364 | 21 | 0.1 | 138 | 0.8 |
| Sub-Boreal Spruce | 92,346 | 10 | 0.1 | 89 | 1.0 |
| Sub-Boreal Pine-Spruce | 22,359 | 2 | 0.1 | 33 | 1.5 |
| Boreal White And Black Spruce | 153,367 | 12 | 0.1 | 140 | 0.9 |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Notes: Data were not available for all species of conservation concern. This table is based on information for 783 out of a total of 1,169 species of conservation concern (mosses were excluded due to lack of information). A species can occur in more than one biogeoclimatic zone.
Boldface indicates biogeoclimatic zone is of conservation concern. The Alpine Tundra zone includes the recently created Interior Mountain-heather Alpine, Coastal Mountain-heather Alpine and Boreal Altai Fescue Alpine zones.
Of the 12 biogeoclimatic zones that are not of conservation concern within the province, five each contain more than 100 species of provincial conservation concern (Coastal Western Hemlock, Interior Cedar-Hemlock, Alpine Tundra,a Engelmann Spruce-Subalpine Fir and Boreal White and Black Spruce) and three each have more than 20 species of global conservation concern (Coastal Western Hemlock, Alpine Tundra and Engelmann Spruce- Subalpine Fir). As these zones occupy large areas, the densities of species of conservation concern are lower.
2.2.1.2 proportion of global range for biogeoclimatic zones
For each biogeoclimatic zone, the proportion of its global range that occurs in B.C. was determined using maps covering a number of neighbouring jurisdictions, combined with expert knowledge where the zones were believed to extend beyond the limits of available information.b,147 Proportion of global range is described by seven classes ranging from 1 (Endemic; 100% of global range in British Columbia) to 7 (Low and Localized; <10% of range in British Columbia and occurs over <30% of the province) (Table 5).
Six of the 16 zones have more than 50% of their global range in B.C. (Classes 1-3) (Table 6; Map 4). These six zones collectively cover about one-quarter of the province. B.C. has 70-80% of the global range of the Coastal Douglas-fir zone, one of the province's four zones of conservation concern, which further emphasizes B.C.'s importance to its conservation. Two zones - the Sub-boreal Pine-Spruce and the Sub-boreal Spruce - are endemic to B.C., meaning they are found nowhere else in the world. Both are forested ecosystems located in the north-central part of the province.
Table 5. Proportion Of Global Range Classification For Ecosystems And Species.
| Global Range Class | Definition (Percent Of Global Range, Area Or Population That Occurs In B.C.) | |
| 1 | Endemic | 100% of global range |
| 2 | Very High | 75-99% of global range |
| 3 | High | 51-74% of global range |
| 4 | Moderately High | 30-50% of global range |
| 5 | Intermediate | 11-29% of global range |
| 6 | Low and Widespread | <10% of global range, and occurs over >30% of the province |
| 7 | Low and Localized | <10% of global range, and occurs over <30% of the province |
Source: Adapted from Bunnell, F., L. Kremsater and I. Houde. 2006. Applying the Concept of Stewardship Responsibility in British Columbia. Biodiversity BC, Victoria, BC. 188pp. Available at: www.biodiversitybc.org.
a Alpine Tundra, a previous classification, was recently split into three separate alpine zones. Due to the unavailability of complete species distribution data for the three new zones, all are reported under the Alpine Tundra zone.
b Ideally, information on the condition of the biogeoclimatic zones in areas outside the province would have been considered (particularly regarding ecosystem conversion); however, those data were not readily available.
2.2.1.3 Shared Ecosystems
Except for the two endemic zones, Sub-boreal Spruce and Sub-boreal Pine-Spruce, all of the province's biogeoclimatic zones are shared with neighbouring jurisdictions (Table 6). For example, the Interior Douglas-fir zone is distributed across British Columbia, Alberta, Montana, Idaho, Washington and Oregon.
2.2.1.4 Status Of Ecological Communities
Ecological communities are ecosystems classified at a much finer resolution than biogeoclimatic zones. The same community can occur in more than one zone. To date, 611 ecological communities have been described in B.C. (Table 7).148 Although not all ecological communities in B.C. have been described, the current list represents a majority of the province's ecological communities.149 Ecological community classification is the most incomplete for alpine ecosystems, but this is a focus of current classification work.150
Of the ecological communities described in B.C., 532 (87%) have had their provincial conservation status assessed and 340 (56% of the total number described) are of provincial conservation concern. As with classification, the majority of the ecological communities that have not been assessed are in alpine ecosystems.151
Table 7. Provincial Conservation Status Of Ecological Communities In B.C. By Biogeoclimatic Zone.
| Biogeoclimatic Zone | Number Of Communities Described | Number Of Communities Assessed | Number Of Communities Of Provincial Conservation Concern | Percent Of Communities That Are Of Provincial Conservation Concern |
| Coastal Douglas-Fir | 36 | 36 | 35 | 97% |
| Bunchgrass | 30 | 30 | 28 | 93% |
| Ponderosa Pine | 29 | 29 | 27 | 93% |
| Coastal Western Hemlock | 128 | 128 | 106 | 83% |
| Interior Douglas-Fir | 87 | 87 | 71 | 82% |
| Sub-Boreal Spruce | 92 | 83 | 56 | 61% |
| Interior Cedar-Hemlock | 89 | 75 | 46 | 52% |
| Sub-Boreal Pine-Spruce | 38 | 34 | 19 | 50% |
| Montane Spruce | 66 | 57 | 31 | 47% |
| Boreal White And Black Spruce | 52 | 41 | 13 | 25% |
| Engelmann Spruce-Subalpine Fir | 149 | 99 | 31 | 21% |
| Mountain Hemlock | 43 | 22 | 8 | 19% |
| Spruce-Willow-Birch | 21 | 1 | 1 | 5% |
| Coastal Mountain-Heather Alpine | 23 | 1 | 1 | 4% |
| Interior Mountain-Heather Alpine | 39 | 2 | 1 | 3% |
| Boreal Altai Fescue Alpine | 53 | 3 | 1 | 2% |
| Province | 611 | 532 | 340 | 56% |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Notes: Boldface indicates biogeoclimatic zone is of conservation concern. Some ecological communities occur in more than one biogeoclimatic zone.
Figure 8: Loss of Idaho fescue- bluebunch wheatgrass ecosystem in the Okanagan Valley since 1800.
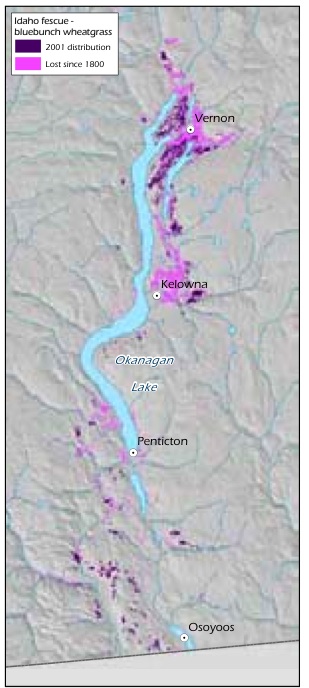
Source: Prepared for this report with data from T. Lea.
As might be expected, the percentage of ecological communities of conservation concern is relatively high in the four biogeoclimatic zones that are of provincial conservation concern (see Section 2.2.1.1, p. 30). It is notable that every biogeoclimatic zone has at least one ecological community in this category. The Coastal Western Hemlock zone stands out as the biogeoclimatic zone with the greatest number of ecological communities of concern (106). It also has the highest percentage (83%) of ecological communities of concern among the 12 zones that are not of provincial conservation concern.
Global conservation status has been assessed for only 113 ecological communities (18% of the total number described).152 This is due to the lack of compatible descriptions of ecological communities between jurisdictions, although work is currently underway to address this issue.153
Text Box 5. Case Study: Loss Of Grassland Ecological Communities In The Okanagan And Lower Similkameen Valleys
Ecosystem conversion of grasslands has been very extensive in the Ponderosa Pine and Bunchgrass biogeoclimatic zones. For example, historical mapping shows that 77% of the Idaho fescue (Festuca idahoensis spp. idahoensis) - bluebunch wheatgrass ecosystem and 68% of the antelope-brush (Purshia tridentata) /needle-and-thread grass ecosystem have been lost to agriculture and urban and rural development in the Okanagan Valley over the past 100 years (Table 8, Figure 8, Figure 9).154 Excessive domestic livestock grazing, off-road recreational vehicles and invasive alien species continue to degrade much of the remaining grasslands in these areas.155 Grasslands are concentrated in the Ponderosa Pine, Bunchgrass and Interior Douglas-fir zones, which is one of the reasons these zones are home to a disproportionate number of B.C.'s terrestrial species of conservation concern (see Table 4, p. 34). Grasslands were rare in B.C. at the time of European contact and have since become rarer because they are found in areas that are attractive for development (e.g., low-elevation areas in the southern part of the province) and are therefore subjected to a high level of ecosystem conversion, as well as fire suppression which results in forest encroachment.
Figure 9: Loss of antelopebrush /needle-and-thread grass ecosystem in the Okanagan Valley since 1800.
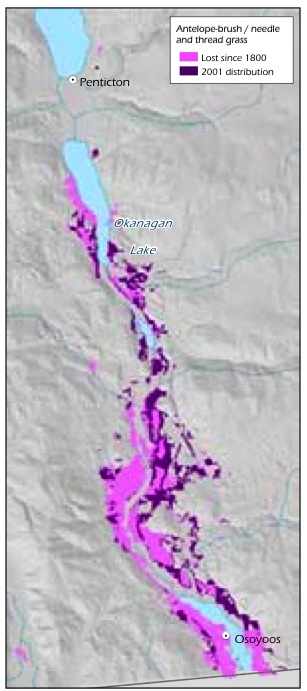
Source: Prepared for this report with data from T. Lea.
Table 8. Historical Loss Of Grassland Ecological Communities In The Okanagan Valley Between 1800 And 2005.
| Grassland Ecosystem Type | 1800 | 1938 | 2005 | Percent Of Ecosystem Lost |
| Water birch /roses | 14,629 | 4,557 | 1,207 | 92% |
| Idaho fescue-bluebunch wheatgrass | 19,253 | 8,657 | 4,395 | 77% |
| Antelope-brush /needle-and-thread grass | 9,905 | 7,325 | 3,160 | 68% |
| Black cottonwood /water birch | 8,111 | 5,176 | 2,864 | 63% |
| Ponderosa pine-bluebunch | 15,149 | 11,471 | 7,172 | 53% |
| wheatgrass gentle slope forest | ||||
| Cattail marsh | 430 | 387 | 257 | 41% |
| Big sagebrush shrub-steppe | 12,233 | 10,314 | 8,266 | 33% |
Source: Lea, T. 2007. Historical (pre-European settlement) ecosystems of the Okanagan and lower Similkameen valleys - applications for species at risk. Saving the Pieces - Restoring Species at Risk Symposium, June 14-16, 2007, Victoria, BC.
Text Box 6. Garry Oak Ecosystems Of The Coastal Douglas-Fir Zonea
Garry oak ecosystems are found within the Coastal Douglas-fir biogeoclimatic zone, one of B.C.'s four zones of conservation concern and the only one of these four for which B.C. has a majority of the global range. Within Canada, Garry oak ecosystems occur only on southeastern Vancouver Island and the southern Gulf Islands, and in two isolated sites in Vancouver. This is one of the ecosystem types of greatest conservation concern in B.C., primarily due to ecosystem conversion resulting from urbanization and agriculture. 156,157 About 10% of the original area that was Garry oak meadow in the mid 1860s still remains, mostly in fragmented remnants that are often dominated by invasive alien species such as Scotch broom (Cytisus scoparius), Himalayan blackberry (Rubus armeniacus), English ivy (Hedera helix) and a variety of non-native grasses and weeds. 158,159 Less than 5% of the original ecosystem remains in near-natural condition (Figure 10).
Garry oak and related ecosystems are home to species of conservation concern such as the sharp-tailed snake (Contia tenuis) and Macoun's meadowfoam, a critically endangered plant for which the province has a majority of the global range.160,161 The potential range of Garry oak ecosystems could expand with climate change,162 but it is uncertain whether the associated native plants will be able to compete with the many alien species now found on Vancouver Island without extensive and costly human intervention.
a Garry oak ecosystems include a group of ecological communities such as the Garry oak - arbutus ecological community.
Figure 10: Past and present distribution of Garry oak ecosystems of southern Vancouver Island and the Gulf Islands.
Source: Lea, T. 2006. Historical Garry oak ecosystems of Vancouver Island, British Columbia, pre-European contact to the present. Davidsonia 17(2): 34-5. Available at: www.davidsonia.org/bc_garryoak.
2.2.2 freshwater ecosystems: major drainage areas
Fresh water is an essential ingredient for life on earth. Most fresh water is frozen or underground, locked either in polar ice caps and permafrost or in underground aquifers, many with recharge times of thousands of years.163 Rivers, lakes, wetlands, soil moisture and water vapour together hold 0.01% of the planet's total water supply (including salt water) and just under 0.4% of the world's fresh water.164 British Columbia has 25% of Canada's supply of flowing fresh water.165
Accessible fresh water in lakes, streams, reservoirs and wetlands provides vital habitat for a disproportionate number of B.C.'s species, including a wide variety of plants, fish, mussels, crayfish, snails, reptiles, amphibians, insects, micro-organisms, birds and mammals that live in, on and around water. Approximately 25% of the species of vertebrates, invertebrates and vascular plants that have been assessed in B.C. are associated with freshwater ecosystems (see Section 2.3.2.1, p. 59). In addition to providing water, food, habitat, and physical, chemical and hydrologic processes, freshwater ecosystems are required for life cycle stages of many organisms, such as salmon (for spawning) and dragonflies (for larval development).
Freshwater ecosystems also provide humans with many essential services. Freshwater ecosystems are highly variable and dynamic. They interact closely with adjacent riparian areas and nearshore communities, sharing physical habitats and ecological and environmental processes, and are highly sensitive to the effects of climate change.
2.2.2.1 conservation status of major drainage areas
To assess the status of freshwater ecosystems, Major Drainage Areas (MDAs) were examined.166,167 With the exception of the Coastal Major Drainage Area, each of B.C.'s nine MDAs encompasses the drainage basin of a major river system in the province (Map 5). The Coastal MDA comprises many small coastal rivers and streams that drain directly into the Pacific Ocean. According to an assessment of conservation status (using the same methods used for biogeoclimatic zones in Section 2.2.1.1, p. 30),168 four of the nine MDAs are of conservation concern (Table 9). The Columbia River drainage, which is highly impacted by dams, is ranked as imperilled. The Fraser River drainage, which includes the highly populated Fraser Valley, is ranked as imperilled/vulnerable.
Table 9. Provincial Conservation Status Of Major Drainage Areas In B.C.
| Major Drainage Area | Conservation Status | Total Area (Km2) | Percent Of Province |
| Columbia | Imperilled (S2) | 102,798 | 11% |
| Fraser | Imperilled/Vulnerable (S2s3) | 231,459 | 25% |
| Coastal | Vulnerable/Apparently Secure (S3s4) | 164,115 | 17% |
| Mackenzie | Vulnerable/Apparently Secure (S3s4) | 278,667 | 30% |
| Taku | Apparently Secure/Secure (S4s5) | 16,585 | 2% |
| Stikine | Apparently Secure/Secure (S4s5) | 49,631 | 5% |
| Yukon | Apparently Secure/Secure (S4s5) | 24,950 | 3% |
| Skeena | Apparently Secure/Secure (S4s5) | 54,401 | 6% |
| Nass | Secure (S5) | 21,530 |
Source: Kremsater, L. 2007. Draft S Ranks and Surrogate G Ranks for BEC Zones and Draft S Ranks for Ecoprovinces and Major Drainage Areas of B.C.: Preliminary Rankings for Informing the Biodiversity Status Report and Action Plan. Biodiversity BC, Victoria, BC. 64pp. Available at: www.biodiversitybc.org.
Notes: Boldface indicates the Major Drainage Area is of conservation concern.
Text Box 7. Lost Streams In The Lower Fraser Valley
The Lower Fraser Valley (LFV) has been considerably altered by human activity over the past 100 years. Large areas of land have been modified for agricultural use, urban and industrial centres or a variety of other purposes. This conversion of ecosystems to other uses has caused heavy damage to streams that at one time supported salmon and other fish. Damage has been caused by destruction of streamside vegetation, water diversion, stream channelization and pollution; and many streams have been effectively lost (i.e., they no longer exist as surface waterways), as a result of being drained, filled, culverted and/or paved over.
A 1997 survey of the LFV (from the Strait of Georgia inland to Hope, and from the North Shore mountains south to the United States border) found that of the 779 streams classified (excluding the Fraser River mainstem and estuary), 86% were either lost, endangered or threatened (Figure 11a).169 The majority of the 14% that remain as wild streams are outside the developed area and have low value for fish because they are inaccessible, high-gradient mountain streams. The survey determined that 117 streams, many of them salmon-bearing, had been lost since the 1860s. All of the lost streams were originally in the area of the LFV that is now occupied by humans (Figure 11b). The Lower Fraser Valley is the spawning habitat for 66% of the wild coho salmon in the Fraser River system.170
Figure 11: Status of streams in the Lower Fraser Valley in 2007
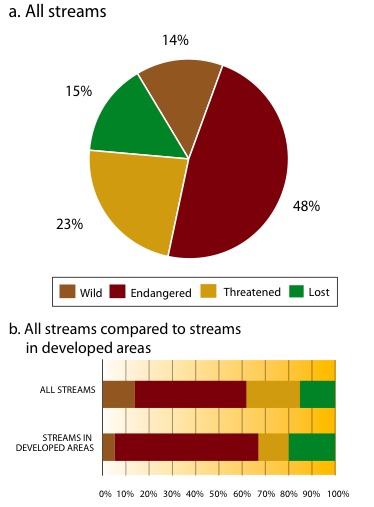
Source: Precision Identification Biological Consultants. 1998. Wild, Threatened, Endangered and Lost Streams of the Lower Fraser Valley: Summary Report 1997. Fraser River Action Plan, Vancouver, BC. 58pp. Available at: www-heb.pac.dfo-mpo.gc.ca/maps/loststrm/loststreams_e.htm. Notes: As the identification of historic streams that have been lost contains an element of uncertainty, the number of lost streams is considered a conservative approximation. Eight impact criteria were developed to assess the status of other streams in the Lower Fraser Valley. A threatened stream meets one impact criterion; an endangered stream meets more than one impact criterion; and a wild stream is not significantly impacted by any criteria, but is not necessarily pristine.
2.2.3 Ecosystems That Overlap The Marine Realm
The coastal zone, where land and ocean meet, is a diverse and productive environment that some consider to be a separate realm.171 There is an exchange of nutrients and energy between the realms through processes such as salmon migration from marine to freshwater ecosystems (see Section 2.5.1.3-F, p. 121) and upland sediment transport (see Section 2.5.1.4-F, p. 131). With a provincial coastline of approximately 29,000 km,172 the area of overlap between marine, terrestrial and freshwater ecosystems in B.C. contains a rich assemblage of ecosystems of wide-reaching importance to the province's biodiversity. This section focuses on intertidal ecosystems and estuaries.
Figure 12:
The intertidal zone. Due to the influences of the land and sea, the intertidal zone contains a rich assemblage of biodiversity and is highly productive.
Illustration: Soren Henrich.
2.2.3.1 Intertidal
The intertidal zone represents the area between the mean high tide line and the mean low tide line, or zero tide, where the benthic substrate is regularly exposed through tidal action (Figure 12). Above the intertidal zone is the supratidal zone - the area below terrestrial trees and shrubs, which contains salt-tolerant grasses and sedges and is influenced, but not dominated, by marine processes such as wave splash, wind-generated storm surge and storm deposits of large woody debris. Below the intertidal zone is the subtidal zone, where the benthic substrate below the lowest normal tide is permanently covered by water. Subtidal community structure is influenced by a number of physical factors (e.g., depth, substrate, salinity, water temperature, wave action, currents, upwellings and light) and biological factors (e.g., larval settlement and dispersal characteristics, predation, productivity and prey availability).
The intertidal zone is regularly exposed to air, wind, sun, rain and sea water as the tide moves in and out, so animals and plants that live in this zone have to adapt to an ever-changing environment. Both biotic and abiotic processes act to maintain the diversity of organisms. For example, in rocky intertidal communities, mussels (Mytilus spp.) tend to exclude all functionally similar organisms that are potential competitors, but ochre sea stars (Pisaster ochraceus) prey on the mussels and prevent them from dominating. Wave action has a similar effect, controlling the most competitive species through desiccation or battering by debris. Vertical zonation of organisms occurs in the intertidal based on the tolerance of each species to desiccation, changes in salinity and light, wave exposure, competition and predation.173
The entire B.C. shoreline has been mapped and classified using both physical and biological mapping components (Table 10).174,175 In a subsequent analysis, estuaries were identified as the most productive habitat, followed by semi-exposed-immobile and current-dominated channel habitats.176 Instances of high species richness were found in all habitat classes except bare beaches and protected shorelines (habitat types 5, 7 and 9), with the majority of instances in exposed to semi-protected immobile habitats along the west coast of Vancouver
Table 10. Habitat Types Used In B.C. Biophysical Shorezone Mapping.
| Habitat Type Class | Habitat Type | Substrate Category |
| 1 | Very exposed-immobile | Bedrock |
| 2 | Exposed immobile | Bedrock |
| 3 | Semi-exposed-immobile | Bedrock/Boulder |
| 4 | Semi-protected-immobile | Bedrock/Boulder |
| 5 | Protected & very protected-immobile | Bedrock/Boulder |
| 6 | Semi-protected-partially mobile | Boulder/Cobble/Pebble |
| 7 | Protected & very protected-partially mobile | Boulder/Cobble/Pebble |
| 8 | Estuaries | Fines/Organic |
| 9 | Bare beaches | Sand/Pebble/Cobble |
| 10 | Current-dominated channels | Bedrock, Sediment or combinations |
| 11 | Hanging lagoons, brackish lakes | Bedrock, Sediment or combinations |
Source: Morris, M., D. Howes and P. Wainwright. 2006. Methodology for Defining B.C. Intertidal ShoreZone Habitats and Habitat Values for the B.C. Oil Spill Shoreline Sensitivity Model. B.C. Ministry of Agriculture and Lands, Victoria, BC. 47pp.
Island, the north and central mainland coast and on Haida Gwaii/Queen Charlotte Islands. Shorelines that are immobile and protected (habitat types 4 and 5) together accounted for about half of the B.C. coast. Estuaries, bare beaches and hanging lagoons were the rarest habitat types, and the majority of these habitats were found on the central and north coast.
Species present in the intertidal are well described compared to pelagic species, because the intertidal zone is relatively accessible. They include many terrestrial species that forage in the intertidal. For example, the diet of coastal black bears includes invertebrates such as shore crabs (Hemigrapsus spp.), porcelain crabs (Petrolisthes spp.), mussels, barnacles (Balanus spp.), isopods (e.g., Idotea spp.) and sea stars, as well as gunnels (e.g., Pholis spp.).177
The intertidal zone has been important to humans in B.C. for generations, historically providing a large portion of the diet of coastal First Nations. Because of the relative ease of access, the intertidal is subject to ecosystem conversion and degradation through human activities, including exposure to contaminants such as persistent organic pollutants, heavy metals, oils and hydrocarbons, and excess nutrients (e.g., excess nitrogen runoff from agriculture or nutrients from sewage resulting in eutrophication). The greatest challenge for the future, however, may be climate change and the anticipated rise in sea level. Introduced species are also a significant threat to intertidal ecosystems.
2.2.3.2 Estuaries
An estuary is generally defined as a partially enclosed body of coastal water, where salt water is measurably diluted by mixing with river runoff. In British Columbia it is estimated that there are more than 440 estuaries occupying approximately 75,000 ha along 2.3% of the length of the coast, with most estuaries ranging in size from 1-10 ha.178,179 Locations of the larger estuaries in B.C. are known and mapped (Figure 13).
The key feature of an estuary is that fresh water meets the salt water of the sea, resulting in brackish water. As a result, estuaries are characterized by salinity rather than geography. When river runoff reaches the salt water there is not an immediate mixing of the two. Rather, the fresh water floats on or near the surface, forming a freshwater plume, while the salt water, having a higher density due to higher dissolved solids, remains below the fresh water, forming a zone sometimes referred to as the saltwater wedge or salt-wedge (Figure 14). The amount of mixing defines different types of estuaries (see Section 2.5.1.4-G, p. 133).
Estuaries comprise a number of identifiable habitat types, such as intertidal flats, marshes/swamps, rivers/lakes, and islands. They present a good example of how ecosystems and ecosystem function are influenced by abiotic components, including seasonal variation in temperature, wave energy, type and rates of sedimentation (turbidity), and timing and volume of freshwater inputs.
The most extensive estuaries are found where the coastline is relatively flat and the sediments brought by the river build up slowly over a wide area and a long time. As described in Section 1.4 (p. 15), estuaries in B.C. are relatively young in geological terms. B.C.'s largest estuary is the Fraser River estuary, with a mapped area of over 21,000 ha and a sphere of influence that spans the Strait of Georgia.180 Its significance is recognized internationally,181,182 but all estuaries make the same kinds of contributions to sustaining biodiversity, albeit at a smaller scale.
Estuaries are nutrient sinks, trapping nutrients from the ocean, land and rivers that are, in turn, dispersed throughout the estuary by tidal movement, wind and currents. The constant mixing creates a productive environment, used by an estimated 80% of all coastal wildlife: for foraging by many species of waterfowl and other birds; and as breeding or rearing grounds by some fish species.183 Estuaries can also sequester and detoxify waste. The influence of estuaries extends beyond their immediate surroundings; nutrients generated in estuaries provide food for many pelagic marine species. Estuaries are of critical importance to the survival of Pacific salmon, particularly juveniles, for reasons that include the provision of nutrients (the fresh water as a source and the saltwater wedge concentrating nutrients) and habitat (diverse habitat types, refuges from predators); most significantly, their low salinity is important to anadromous fish as they make the transition between the marine and freshwater realms. 184
Because estuaries are productive ecosystems and offer easy access to the sea, humans have long been drawn to settle and develop infrastructure near them, leading to ecosystem conversion and degradation, environmental contamination (of both water and sediment), disturbance and alien species introductions.185 Section 2.5.1.4-G (p. 133) provides more information on threats to estuaries.
Figure 13: Locations of mapped estuaries in B.C. Source: Adapted from Ryder, J.L., J.K. Kenyon, D. Buffett, K. Moore, M. Ceh and K. Stipec. 2007. An Integrated Biophysical Assessment of Estuarine Habitats in B.C. to Assist Regional Conservation Planning. Canadian Wildlife Service, Pacific and Yukon Region, Delta, BC. Technical Report Series No. 476.
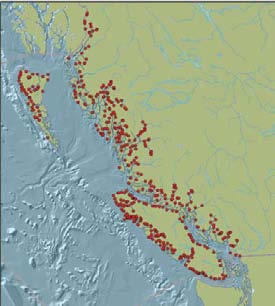
Figure 14: The interface between the freshwater plume and the saltwater wedge.
Source: Adapted from Fisheries and Oceans Canada. 2007. Estuaries: The Physical Environment. Available at: www.glf.dfo-mpo.gc.ca/os/bysea-enmer/estuaries-estuaires-e.php. illustration: Soren Henrich.
2.2.4 Data Gaps
Within B.C., the classification and mapping of ecological communities is incomplete. The major gaps are in the alpine biogeoclimatic zones and in small communities such as vernal pools, rock outcrops and avalanche tracks.186,187
There is no province-wide data source to update the structural stage of ecosystems. For example, there are gaps for forest age in some protected areas and Tree Farm Licenses.188 Classification and mapping of freshwater ecosystems is far less advanced than ecosystem mapping in the terrestrial realm. A recent attempt has been made at classifying freshwater systems (drainage units, watersheds, lakes and rivers), but it has not yet been widely adopted.189
Global status assessments have not been completed for the majority of ecological communities. The global status assessment of ecosystems is compromised by differences between ecological classification in B.C. and adjacent Canadian and American jurisdictions, lack of comparisons of these ecological classifications and limited information on impacts and trends.190 This lack of information also impacts the ability to determine what proportion of the global range of ecological communities occurs in B.C.
Information on trends for ecosystems is very incomplete. For example, baseline information on the historic extent of ecosystems is limited to a small number of ecosystems in specific areas.191
Although climate change will cause species distributions to shift, ecosystems will not move.192 Instead they will change in terms of their species composition, as well as their structure and function. As a result, any ecosystem classification scheme will become obsolete over time.193 This places added importance on the use of units that will not change as the climate changes, such as terrain units, which are based on topography and soils. However, the provincial coverage of terrain units is incomplete.194
2.3 Diversity of Species in British Columbia
Species interact within ecosystems, performing essential ecological functions necessary for life on earth (see Section 1.2, p. 10). This section summarizes information on the status of about 3,800 native species,a including more than 2,000 vascular plants, 563 vertebrate animals, 423 invertebrates and over 729 non-vascular plants. These are the species we know the most about, but they represent only a fraction of the approximately 50,000 species (not including single-celled organisms) that exist in B.C.195
a Includes mammals, birds, freshwater fish (including anadromous species such as salmon), reptiles and turtles, amphibians, butterflies and skippers, dragonflies and damselflies, non-marine molluscs, flowering plants (monocots and dicots), ferns and fern allies, mosses and conifers. Only full species with scientifically accepted taxonomic names are included. Alien species are not included, nor are 'accidental' species (i.e., those that occur in B.C. infrequently and unpredictably, as B.C. is outside their usual range).
Information about most species in British Columbia is limited. Surveys and incidental observations are often sporadic, inconsistent and/or concentrated along roads and in areas of higher human population. Parts of the province have never been surveyed and a number of taxonomic groups have never been assessed (see Appendix B, p.232).a
The number of species included in each of the analyses presented in this section varies according to the availability of data (Table 11). For the species richness analysis, non-marine molluscs and mosses were excluded due to concerns that the available data were overly biased by survey effort, and the analysis of birds was limited to passerines (perching birds) due to the lack of data for other types of birds. Also for the species richness analysis, there were some species in other groups for which no recent documented occurrences were available (records prior to 1961 were excluded for all groups). Mosses were excluded from the realm overlap analysis due to the lack of expertise to assign them to a realm or realms. Other differences in the number of species considered for each analysis are generally very minor and are due to varying species lists.
2.3.1 Richness
Species richness is one common measure of biodiversity, calculated as the number of species in an area of interest. For this analysis of species richness, the province was divided into a grid of 1,208 squares on a map.b The number of species recorded in each grid square was then calculated from computerized location data.196 The species groups assessed were those from the best-studied groups of plants and animals for which adequate computerized location data (recorded between 1961 and 2006) were available.
Map 6 shows patterns of species richness across the province for 2,640 vertebrates, invertebrates and vascular plants. Species richness varies markedly across the province and is highest in the south of the province and on Vancouver Island, which are also areas of highest human population density. The biogeoclimatic zones with the highest species richness are Ponderosa Pine, Coastal Douglas-fir, Bunchgrass and Interior Douglas-fir, all ecosystems of conservation concern (see Section 2.2.1.1, p. 30).
The data are biased because surveys and incidental observations most often occur close to roads. For example, high species-richness points occur along the Alaska Highway north of Fort St. John at Pink Mountain, known for its wildflowers and rare Arctic butterflies, and at Liard Hot Springs, a provincial park. Some of the large areas depicted as having low species richness are the most inaccessible in the province (e.g., north of Spatsizi Provincial Park) and have not been well surveyed. Such is also the case along much of B.C.'s rugged coastline.
a Conservation status is only assessed for entire taxonomic groups subject to the availability of information; it is not focused on species that are suspected to be of conservation concern.
b Grid squares correspond to 1:50,000-scale map sheets and range in size from 1,030 to 780 km2 (the size decreases moving from south to north). For more information, see the National Topographic Service website: http://maps.nrcan.gc.ca/topo_e.php.
Table 11. Number Of Species Considered For The Analyses Of Species Richness, Conservation Status, Proportion Of Global Range And Realm Overlap, By Taxonomic Group.
| Taxonomic Group | Species Richness Analysis | Conservation Status Analysis | Proportion Of Global Range Analysis | Realm Overlap Analysis |
| Birds | 187 | 353 | 352 | 349 |
| Conifers | 25 | 26 | 26 | 26 |
| Flowering Plants (Monocots) | 525 | 552 | 556 | 549 |
| Flowering Plants (Dicots) | 1,339 | 1,404 | 1,403 | 1,402 |
| Non-marine Molluscs | 0 | 157 | 157 | 157 |
| Mosses | 0 | 729 | 760 | 0 |
| Ferns and Fern Allies | 103 | 111 | 111 | 111 |
| Reptiles and Turtles | 12 | 14 | 14 | 13 |
| Amphibians | 20 | 20 | 20 | 20 |
| Mammals | 102 | 109 | 109 | 109 |
| Freshwater Fish | 70 | 67 | 67 | 67 |
| Dragonflies and Damselflies | 85 | 86 | 86 | 86 |
| Butterflies and Skippers | 172 | 180 | 180 | 180 |
| Total | 2,640 | 3,808 | 3,841 | 3,069 |
Source: Prepared for this report.
Despite these limitations, the observed pattern is consistent with the global pattern of decreasing species richness at higher latitudes197 and elevations.198
The patterns of high species richness on the province's large coastal islands (Vancouver Island and Haida Gwaii/Queen Charlotte Islands) are notable. As a rule, islands have lower diversity than areas of equal size on the adjacent mainland, with decreasing disparity as island size increases and distance from the mainland decreases.199 Although this seems to hold true for B.C.'s smaller islands, which have low species richness due to little variation in habitat, the province's larger islands are species-rich relative to the adjacent mainland, likely because they have a mild, moist climate, large elevational range, variation in climate and close proximity to the mainland, and because portions of these islands were refugia during the last glaciation (see Section 2.4.1.3, p. 78).
2.3.2 Conservation Status
Conservation status rankings (Table 12) were compiled for 13 of the best-studied groups of native plants and animals based on information current to 2007.200 Criteria for these rankings included rarity, population size and trends, and the level of threat from human activity. For the purposes of this analysis, species falling into the categories Extirpated (X), Historical (H), Critically Imperilled (1), Imperilled (2) and Vulnerable (3) were considered to be species of conservation concern (also termed 'at risk') in British Columbia. Information was compiled at two scales: global (G), indicating the status of a species in its worldwide range, and subnational/provincial (S), indicating the status of a species within B.C.
The conservation status of a species may vary according to the area considered. For example, the sharptailed snake has a global conservation status of G5, indicating its secure status across its entire range, but a provincial conservation status rank of S1 to convey its limited occurrence and high level of imperilment in British Columbia. The provincial status ranking of a species can never be lower (i.e., more secure) than its global status ranking.
Of the 3,808 native species in British Columbia for which conservation status has been assessed, 91% are globally secure (G5) or apparently secure (G4), whereas only 54% are provincially secure (S5) or apparently secure (S4) (Table 13). In B.C., 233 species (6%) are of global conservation concern and 1,640 species (43%) are of provincial conservation concern. The proportion of species in B.C. that are of global conservation concern is relatively low; of the 32,487 native species in the U.S. and Canada assessed by NatureServe, 12,700 (39%) are considered to be of global conservation concern.201 The high proportion of species of provincial conservation concern reflects, in part, the high number whose habitat in B.C. was rare even before European contact and the concentration of ecosystem conversion in these areas; for example, in some warm, dry, low-elevation areas of southern B.C. (see Section 3.2.1, p. 159).
Table 12. Conservation Status Ranks For Species In B.C.
| Rank | Definition | Description |
| x | extinct or presumed extirpated | not located despite intensive searches and no expectation of rediscovery. |
| H | Historical | possibly extinct or extirpated; known only from historical occurrences, but still hope of rediscovery. |
| 1 | critically imperilled | at very high risk of extirpation or extinction due to extreme rarity (often 5 or fewer populations), steep declines or other factors, making the species especially susceptible to extirpation or extinction. |
| 2 | imperilled | at high risk of extirpation or extinction due to very restricted range, few populations (often 20 or fewer), steep declines, or other factors. |
| 3 | Vulnerable | at moderate risk of extirpation or extinction due to a restricted range, relatively few populations (often 80 or fewer), recent and widespread declines, or other factors. |
| 4 | Apparently Secure | Uncommon but not rare, and usually widespread in the range. Some cause for long-term concern. |
| 5 | Secure | Common or very common, and widespread and abundant. Not susceptible to extirpation or extinction under current conditions. |
| NA | Not Assessed | Species whose pattern of occurrence in the province is not compatible with the assessment process. |
| NR | Not yet Ranked | Rank is not yet assessed. |
| U | Unrankable | Suitable information is not available for ranking. |
Source: Adapted from Anions, M. 2006. Global and Provincial Status of Species in British Columbia. Biodiversity BC, Victoria, BC. 16pp. Available at: www.biodiversitybc.org.
Notes: For analyses in this report, range ranks (given when not enough information is available to score a specific rank) are rounded to the higher rank (e.g., S2S3 is rounded to S2; S2S4 is averaged to S3). Boldface indicates that species with these ranks are of conservation concern.
Three percent of the species considered are not assessed (NA), not yet ranked (NR) or are unrankable (U). Species not assessed are those whose pattern of occurrence in the province is not compatible with the assessment process, such as some migratory species that do not breed in B.C. (e.g., short-tailed albatross [Phoebastria albatrus]).
Within the taxonomic groups assessed, the non-marine molluscs have the highest proportion of species of global conservation concern (22%), followed by the mosses (12%), ferns and fern allies (12%) and reptiles and turtles (7%) (Figure 15). The groups with the highest numbers of species of global conservation concern are the mosses (88 species), dicots (58 species) and non-marine molluscs (34 species). Map 7 shows the distribution of the 233 species of global conservation concern for which computerized location data were available.
Table 13. Summary Of B.C. Species Assessed For Global And Provincial Conservation Status.
| Conservation Status Rank | Global Number of Species | Global Percentage of Species | Provincial Number of Species | Provincial Percentage of Species |
| Extinct or Extirpated (GX, SX) | 1 | <1% | 14 | <1% |
| Historical (GH, SH) | 1 | <1% | 28 | <1% |
| Critically Imperilled (G1, S1) | 19 | <1% | 301 | 8% |
| Imperilled (G2, S2) | 40 | <1% | 629 | 17% |
| Vulnerable (G3, S3) | 172 | 5% | 668 | 18% |
| Total species of conservation concern | 233 | 6% | 1,640 | 43% |
| Apparently Secure or Secure (G4, S4, G5, S5) | 3,475 | 91% | 2,055 | 54% |
| Not Assessed, Not Ranked, or Unrankable (NA, NR, or U) | 100 | 3% | 113 | 3% |
| Total number of species assessed | 3,808 | 3,808 |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Figure 15: Species of global conservation concern as percent of total number of plant and animal species assessed in B.C.
Notes: Total number of species assessed = 3,808. For each species group, numbers shown represent the number of species assessed as being of global conservation concern and the total number of species in the group (e.g., birds: 10 species of global conservation concern /353 species in total).
Within the taxonomic groups assessed, the mosses have the highest proportion of species of provincial conservation concern (65%), followed by the reptiles and turtles (64%), ferns and fern allies (58%) and dicots (46%) (Figure 16). The groups with the highest numbers of species of conservation concern in the province are the dicots (651 species), mosses (471), monocots (196), birds (70) and ferns and fern allies (64). Map 8 shows the distribution of the 1,640 species of provincial conservation concern for which computerized location data were available.
Figure 16: Species of provincial conservation concern as percent of total number of plant and animal species assessed in B.C.
Notes: Total number of species assessed = 3,808. For each species group, numbers shown represent the number of species assessed as being of provincial conservation concern and the total number of species in the group (e.g., birds: 70 species of provincial conservation concern /353 species in total).
Table 14. Extinct And Presumed Extirpated Species In B.C.
| Taxonomic Group | Scientific Name | Common Name | Conservation Status |
| Birds | Centrocercus urophasianus | Greater sage grouse | Extirpated |
| Coccyzus americanus | Yellow-billed cuckoo | Extirpated (breeding populations) | |
| Ectopistes migratorius | Passenger pigeon | Extinct | |
| Reptiles and Turtles | Actinemys marmorata | Western pond turtle | Extirpated |
| Phrynosoma douglasii | Pigmy short-horned lizard | Extirpated | |
| Butterflies | Limenitis archippus | Viceroy | Extirpated |
| Non-marine Molluscs | Cryptomastix devia | Puget oregonian | Extirpated |
| Vascular Plants | Downingia elegans | Common downingia | Extirpated |
| Epilobium torreyi | Brook spike-primrose | Extirpated | |
| Lepidium oxycarpum | Sharp-pod peppergrass | Extirpated | |
| Lupinus oreganos | Kincaid's lupine | Extirpated | |
| Non-vascular Plants | Micromitrium tenerum | [no common name] | Extirpated |
| Physcomitrium immersum | [no common name] | Extirpated | |
| Pseudephemerum nitidum | [no common name] | Extirpated |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Notes: Kincaid's lupine (Lupinus oreganus var. kincaidii) is the only variety of Lupinus oreganus represented in B.C. and is therefore listed at the species level. The western pond turtle has not been recorded since 1966 and there are only two previous specimen records. It may have been introduced, but there is no concrete evidence to suggest this. Proximity to Puget Sound populations suggests that a B.C. native population was a possibility (Cannings, S.G., L.R. Ramsay, D.F. Fraser and M.A. Fraker. 1999. Rare amphibians, reptiles, and mammals of British Columbia. Wildlife Branch and Resources Inventory Branch, Ministry of Environment, Lands and Parks, Victoria, BC. 198pp.).
Text Box 8. Extinct And Extirpated Species
An extinct species is one that has disappeared from its global range. An extirpated species is one that is no longer found in a given area (i.e., in B.C., for the purposes of this report) despite intensive searches, and for which there is little hope of rediscovery. Extinct species are gone forever, but an extirpated species has the potential to be reintroduced.202 Fourteen species once found in B.C. have been designated extinct or extirpated (Table 14). An additional 28 species are considered historic, meaning there is no verified record of their presence in the past 40 years; although they are possibly extinct or extirpated, rediscovery remains a possibility (see Appendix A, p.231). Extinct and extirpated taxa below the species level (subspecies, populations, varieties) are discussed in Section 2.4.2 (p. 82).
2.3.2.1 Species Of Conservation Concern In The Terrestrial, Freshwater And Marine Realms
For the analysis of species status within the terrestrial and freshwater realms, as well as those that overlap with the marine realm, the number of species associated with each realm was determined by classifying species according to their requirements for terrestrial, freshwater or marine ecosystems for at least one of their life requisites (food, shelter or reproduction). Species that require both marine and freshwater, or both marine and terrestrial, ecosystems were included in the analysis. Exclusively marine species were not included.
The assessment of 3,079 species of vertebrates, invertebrates and vascular plantsa showed that 2,612 species (85%) are associated with terrestrial ecosystems, 769 (25%) with freshwater ecosystems and 152 (5%) with marine ecosystems (Table 15).b Species that require more than one ecosystem type to meet all of their life requisites are counted in each appropriate realm. For example, Merriam's shrew (Sorex merriami) relies only on terrestrial ecosystems for all of its life requisites and is classified as terrestrial, whereas the Pacific water shrew (Sorex bendirii) dens on land and forages in or near water and is classified as both terrestrial and freshwater. Because the marbled murrelet (Brachyramphus marmoratus) nests in old-growth trees in forests, forages for both marine and freshwater prey, and winters at sea, it is counted in all three realms.
a Mosses were excluded due to lack of information.
b Species were assigned to the marine, terrestrial and freshwater realms by J. Cooper, B. Costanzo, A. Eriksson, J. Heron, D. Nagorsen, G. Scudder or L. Warman.
Figure 17: Species of global and provincial conservation concern in the terrestrial, freshwater and marine realms.
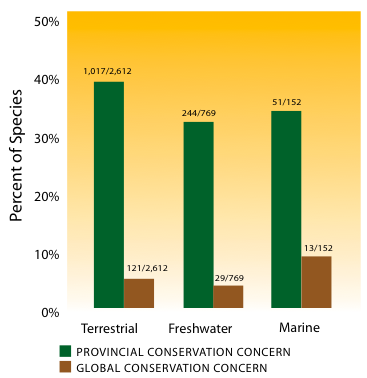
Notes: Total number of species assessed = 3,079 (including vertebrates, invertebrates and vascular plants; excluding mosses). Some species are associated with more than one realm.
Text Box 9. Trends In Conservation Status Of Select Groups Of Species And Subspecies203,204
The B.C. Conservation Data Centre annually reviews the conservation ranks for B.C. species and subspecies. The conservation rank for a species can change because of a genuine improvement or deterioration in the status of the species or for several other reasons. For example, a species rank may be adjusted because the assessor has access to improved information or because a previously unknown population has been discovered.
To determine the true change in status for the groups of species shown in Figure 18, all available species ranks were compared between a point in the 1990s and one in the 2000s. All changes in rank that were due to changes in knowledge about that species, how it was assessed or how it was classified taxonomically were removed.
This analysis of four of B.C.'s best-studied groups shows that for mammals, freshwater fish and vascular plants of highest conservation concern, more species and subspecies have experienced a deterioration in conservation status since the 1990s than have experienced an improvement. The large number of breeding birds with improved status is in large part due to new immigrant species (i.e., species that have entered the province without human assistance). Because of their initial small populations, they were originally ranked as being of high conservation concern, but many of these species have since expanded their ranges, resulting in gradual improvement in their conservation status ranks. As a group, breeding birds also had the largest proportion and largest total number of species whose conservation status deteriorated. Due to their mobility, it is possible that birds respond more rapidly - both positively and negatively - to habitat change and climate change. A majority of species in all four of the groups analyzed showed no change in conservation status during the period examined. Of the species assessed, 1,017 (39%) terrestrial species, 244 (32%) freshwater species, and 53 (34%) marine overlap species are of provincial conservation concern, and 121 (5%) terrestrial species, 29 (4%) freshwater species and 14 (9%) marine overlap species are of global conservation concern (Figure 17).
Figure 18: Species and subspecies with changed conservation status in B.C. since the 1990s.
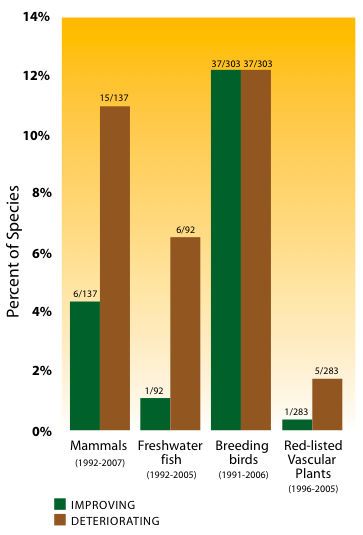
Notes: Period of assessment varies by species group: mammals (1992-2007); freshwater fish (1992-2005); breeding birds (1991-2006); vascular plants of highest conservation concern (1996-2005). Data do not include species introduced by humans. Breeding birds include new immigrant species.
2.3.3 Proportion Of Global Range For Species
The proportion of global range was assessed based on seven classes, ranging from 1 (Endemic; 100% of global range or population in range or population in British Columbia) to 7 (Low and Localized; <10% of range or population in British Columbia and occurs over <30% of the province) (Table 5, p. 35). Species in classes 1-3 (Endemic, Very High and High) have a majority (>50%) of their range, area or population within the province.
Consideration of the proportion of a species' global range in B.C. should be balanced by consideration of how the species is distributed across other jurisdictions. This is particularly important for species that are low and widespread (meaning a low proportion of their global range occurs in B.C., but they still occupy >30% of the province and by extension must have a large global range). For example, fishers (Martes pennanti) are sufficiently widespread that no jurisdiction has more than 10% of the global range.205
Most of the assessments for species were based on the proportion of global range occurring in British Columbia using range maps and available presence or absence information. Although ideally the proportion of the population within a jurisdiction would be used, this could only be approximated for three broad groups of species - birds showing strong seasonal aggregations, some marine mammals that congregate on land (e.g., Steller sea lion [Eumetopias jubatus]), and well-monitored game species - as well as for a few species of conservation concern (e.g., American white pelican [Pelecanus erythrorhynchos], Vancouver Island marmot).
Table 16. Summary Of B.C. Species By Global Range Class.
| Global Range Class | Number Of Species In Range Class | Percent Of Total Species Assessed |
| Endemic (1) | 15 | <1% |
| Very High (2) | 15 | <1% |
| High (3) | 69 | 2% |
| Total number of species with a majority of global range in B.C. | 99 | 3% |
| Moderately High (4) | 189 | 5% |
| Intermediate (5) | 497 | 13% |
| Low and Widespread (6) | 1,249 | 33% |
| Low and Localized (7) | 1,714 | 45% |
| Not ranked | 93 | 2% |
| Total number of species assessed | 3,841 |
Source: Bunnell, F., L. Kremsater and I. Houde. 2006. Applying the Concept of Stewardship Responsibility in British Columbia. Biodiversity BC, Victoria, BC. 188pp. Available at: www.biodiversitybc.org.
The proportion of global range was assessed for 3,841 native species in 13 taxonomic groups (Table 16). Of these, 99 species (3%) have a majority of their global range in the province. Fifteen species, 10 of which are plants, are endemic (Class 1). Only one B.C. endemic species, Newcombe's butterweed (Sinosenecio newcombei), is not of conservation concern.
The groups with the highest proportion of species that have a majority of their global range in B.C. are the conifers (12%), freshwater fish (7%), non-marine molluscs (5%), birds (3%) and amphibians (5%) (Figure 19). The dicots have the highest number of species with a majority of their global range in B.C. (33).
Computerized location data were available for 82 of the 99 species with a majority of their global range in B.C. Species richness for these species is shown in Map 9. Currently, 30 of the 233 B.C. species that are of global conservation concern are also among the 99 species with a majority of their global range in B.C. (Table 17).206 An additional 11 species with a majority of their global range in B.C. are among the 1,640 of provincial conservation concern, but are not of global conservation concern. It should be noted that not all species in the province have been assessed, including most invertebrate groups.
Figure 19: Species with a majority of their global range in B.C. as a percent of the species assessed.
Notes: Total number of species assessed = 3,841. For each species group, numbers shown represent the number of species assessed as having the majority of their global range in B.C. and the total number of species in the group (e.g., birds: 12 species of global conservation concern /352 species in total).
At the species level, B.C. has a very low level of endemism, considering that 5,000 or more species have been recorded elsewhere in global hot spots of plant endemism.207,208 This is consistent with the province's recent history of glaciation. Of the insects, only the dragonflies and butterflies were included in this assessment. Although many other invertebrate species are currently listed as endemic in British Columbia,209 they may not be true endemics and their listing may be due to a lack of collecting and knowledge of their full distribution. Information is also very incomplete on mosses and lichens endemic to the Pacific Northwest and known to occur in B.C.210
Table 17. Species Of Provincial Or Global Conservation Concern With A Majority Of Their Global Range In B.C.
| Scientific Name | Common Name | Global Concern | Provincial Concern | Proportion Of Global Range Class |
| Birds | ||||
| Ptychoramphus aleuticus | Cassin's auklet | G4 | S2S3 (breeding) S4 (non-breeding) |
Very High (2) |
| Freshwater Fish | ||||
| Lampetra macrostoma | Vancouver (or Cowichan Lake) lamprey | G1 | S1 | Endemic (1) |
| Salvelinus confluentus | Bull trout | G3 | S3 | High (3) |
| Mammals | ||||
| Marmota vancouverensis | Vancouver Island marmot | G1 | S1 | Endemic (1) |
| Myotis keenii | Keen's myotis | G2G3 | S1S3 | Very High (2) |
| Sorex bendirii | Pacific water shrew | G4 | S1S2 | High (3) |
| Non-Marine Molluscs | ||||
| Fossaria truncatula | Attenuate fossaria | G3 | S3 | High (3) |
| Fossaria vancouverensis | [no common name] | GH | SH | Very High (2) |
| Hemphillia dromedarius | Dromedary jumping slug | G3G4 | S2 | High (3) |
| Physella wrighti | Hotwater physa | G1 | S1 | Endemic (1) |
| Planorbella columbiensis | Caribou rams-horn | G1G2 | SH | Endemic (1) |
| Pristiloma chersinella | Black-footed tightcoil | G3G4 | S3S4 | High (3) |
| Vascular plants | ||||
| Asplenium adulterinum | Corrupt spleenwort | G3 | S2S3 | Endemic (1) |
| Aster paucicapitatus | Olympic mountain aster | G3 | S3 | High (3) |
| Bidens amplissima | Vancouver Island beggarticks | G3 | S3 | Very High (2) |
| Enemion savilei | Queen Charlotte false rue-anemone | G3G4 | S3S4 | Endemic (1) |
| Erigeron trifidus | Three-lobed daisy | G2G3 | S2 | High (3) |
| Geum schofieldii | Queen Charlotte avens | G2 | S2 | Endemic (1) |
| Impatiens aurella | Orange touch-me-not | G4 | S2S3 | High (3) |
| Isoetes minima | Midget quillwort | G1G2 | S1 | High (3) |
| Vascular plants | ||||
| Ligusticum caldera | Calder's lovage | G3G4 | S3S4 | Very High (2) |
| Limnanthes macounii | Macoun's meadow-foam | G2 | S2 | Endemic (1) |
| Listera convallarioides | Broad-leaved twayblade | G5 | S3S4 | High (3) |
| Saxifraga taylori | Taylor's saxifrage | G3G4 | S3S4 | Endemic (1) |
| Senecio moresbiensis | Queen Charlotte butterweed | G3 | S3 | High (3) |
| Sinosenecio newcombei | Newcombe's butterweed* | G4 | S4 | Endemic (1) |
| Talinum sediforme | Okanagan fameflower | G3 | S2S3 | High (3) |
| Viola biflora | Queen Charlotte twinflower violet | G5 | S3 | High (3) |
| Non-Vascular Plants | ||||
| Andreaea sinuosa | [no common name] | G2 | S1 | High (3) |
| Brotherella roellii | [no common name] | G3 | S3 | Endemic (1) |
| Bryhnia hultenii | [no common name] | G4 | S1 | High (3) |
| Ctenidium schofieldii | [no common name] | G2G3 | S2S3 | Endemic (1) |
| Orthotrichum pulchellum | [no common name] | G4 | S3S4 | High (3) |
| Pohlia cardotii | [no common name] | GU | S2S3 | High (3) |
| Pohlia columbica | [no common name] | G3G5 | S1S3 | High (3) |
| Pohlia pacifica | [no common name] | GU | S1S3 | Endemic (1) |
| Seligeria careyana | [no common name] | G1 | S1 | Endemic (1) |
| Trematodon boasii | [no common name] | G1 | S1 | High (3) |
| Trematodon montanus | [no common name] | G1 | S1 | High (3) |
| Ulota obtusiuscula | [no common name] | GU | S3S4 | High (3) |
| Wijkia carlottae | [no common name] | G2 | S2 | Endemic (1) |
| Zygodon gracilis | [no common name] | G2 | S1 | High (3) |
Sources: Anions, M. 2006. Global and Provincial Status of Species in British Columbia. Biodiversity BC, Victoria, BC. 16pp.; and Bunnell, F., L. Kremsater and I. Houde. 2006. Applying the Concept of Stewardship Responsibility in British Columbia. Biodiversity BC, Victoria, BC. 188pp. Both available at: www.biodiversitybc.org.
Notes: *Newcombe's butterweed is the only B.C. endemic species that is not of conservation concern. It is included here to provide a complete list of endemic species. Only one subspecies (ssp. carlottae) of Viola biflora occurs in B.C., therefore it is included at the species level. All non-vascular plants listed in the table are mosses.
2.3.3.1 Species At The Edge Of Their Range
Of the species that were assessed for the proportion of their global range in B.C., 2,963 (78%) have <10% of their range, area or population within the province (global range classes 6 and 7) (Table 16, p. 61). Most of these species are at the edge of their range in B.C. and are sometimes called 'geographically marginal' or 'peripheral' species (see Section 2.4.1.1, p. 74).
The proportion of the global range that occurs in B.C. is increasing for some species.211 Species range shifts due to climate change are generally expected to be northwards, 212, 213,214,215,216,217 and such shifts are facilitated by the north-south orientation of mountain ranges in B.C. Researchers have also found that mammal species that are reduced to less than 25% of their historic range tend to become limited to the periphery of their range; 74% of those studied showed this trend, and the most common direction of the collapse was from east to west and from south to north.218 This suggests there may be a tendency for species ranges to collapse toward B.C.
2.3.4 Species Overlap: Realm And Jurisdictional
Species and ecosystems transcend lines on maps. Natural processes and human actions in one realm or jurisdiction can have a profound impact on biodiversity in adjacent realms or jurisdictions. Some species, such as the eulachon, marbled murrelet and anadromous salmon, spend portions of their life cycles in different realms. Others, like the Steller sea lion, may transit between the terrestrial and marine realms on a daily basis and between jurisdictions over the course of their life.
2.3.4.1 species that overlap with the marine realm Some species require marine ecosystems in addition to freshwater and/or terrestrial ecosystems in order to live or complete their life cycle. These species have adapted to take advantage of the different structures of the two realms (see Section 2.2.3, p. 45). Marine mammals such as harbour seals (Phoca vitulina) haul out on rocky islets. Seabirds often nest near freshwater lakes or on cliffs, or, like the marbled murrelet, in the tops of trees. Some grasses and sedges are found in brackish tidal marshes, providing habitat for terrestrial and marine species.
Table 18. Terrestrial And Freshwater Species In B.C. That Overlap With The Marine Realm.
| Taxonomic Group | Number Of Overlap Species | Provincial Concern | Global Concern | Number of Species With Majority Of Global Range in B.C. (Classes 1-3) |
| Birds | 77 | 17 | 7 | 2 |
| Freshwater Fish | 8 | 3 | 1 | 1 |
| Mammals | 6 | 2 | 2 | 0 |
| Vascular Plants | 61 | 29 | 3 | 0 |
| Total | 152 | 51 | 13 | 3 |
Source: Prepared for this report.
Notes: For number of species considered, see Table 11, p. 51. For taxonomic groups listed in Table 11 and not listed in this table, there were no species that overlap with the marine realm. Vascular plants include flowering plants (monocots and dicots) and conifers.
Of the 152 species of vertebrates, invertebrates and vascular plants identified as marine overlap species (Table 18), 51 are of provincial conservation concern and 13 are of global conservation concern. The latter include the pink-footed shearwater (Puffinus creatopus), a seabird that is critically imperilled in its global range; the Steller sea lion, which congregates in rookeries, generally on remote rocky islands, for breeding and pupping (see Section 2.5.2.1-B, p. 139); and Alaskan orache (Atriplex alaskensis), an annual herb that grows in saline soils along coastlines and is possibly extirpated from B.C. The three marine overlap species of global conservation concern that have the majority of their global range in B.C. are Barrow's goldeneye (Bucephala islandica), Cassin's auklet (Ptychoramphus aleuticus) and sockeye salmon.
2.3.4.2 Species That Overlap With Other Jurisdictions
Plants and animals do not recognize political boundaries. They may migrate, travel throughout their home ranges, swim along river systems or, in some cases, travel with the wind or attached to other organisms that move from one place to another. Habitats are often intersected by jurisdictional boundaries, which, like the boundary between Canada and the United States, frequently follow non-ecological lines. Even within British Columbia, management responsibility for species and habitats may shift from one entity to another, with overlapping responsibilities.
British Columbia shares its boundaries with seven other jurisdictions: three in Canada - the Yukon, Alberta and the Northwest Territories - and four in the United States - Alaska, Washington, Montana and Idaho. Because B.C. has very few endemic species (see Table 16, p. 61), the province shares almost all of its species with one or more of these other jurisdictions. To complicate matters, some species of butterflies, birds and anadromous fish migrate between British Columbia and distant jurisdictions or have disjunct seasonal distributions.
Using data from NatureServe and WildSpeciesa,219 the status of 140 species of vertebrates (amphibians, birds, freshwater fish, mammals, and reptiles and turtles) of conservation concern in B.C. was compared with their status in adjacent jurisdictions (Table 19). This analysis was limited to vertebrate species because they are the most consistently assessed across jurisdictions and have the greatest amount of data to support the assessments conducted.
Taxonomic groups with the highest numbers of shared species are those that are most mobile, such as birds. The jurisdictions with the highest numbers of shared species are those located south of B.C. This corresponds with the higher levels of species richness in the southern parts of the province.
Most species have been assessed in at least one neighbouring jurisdiction, but only 20% of species are not of conservation concern in any jurisdiction other than B.C., although 60% are not of conservation concern in at least one neighbouring jurisdiction. More than 35% of the species are of conservation concern in all neighbouring jurisdictions in which they are present and have been assessed. Each jurisdiction has some species that are present, but have not been assessed.
a WildSpecies data were used to identify conservation status for species in the Northwest Territories that were not found in the NatureServe database.
Of particular interest are the recorded occurrences of species that are of conservation concern in B.C., yet secure in other jurisdictions. For example, the sage thrasher (Oreoscoptes montanus) is critically imperilled in B.C. and secure in Idaho. This may be the result of a common species from an adjacent jurisdiction expanding its range into B.C., where it establishes small, discrete populations that are of greater conservation concern than the core population. 220 Other possible explanations include species being historically and naturally rare in B.C., or species being impacted by threats in B.C. that are not as prevalent in adjacent jurisdictions.
Several species that are presumed or possibly extirpated (SX, SH) in B.C. are found in Washington, but are all either critically imperilled, imperilled or possibly extirpated in that jurisdiction.221 Only two of the species that are presumed or possibly extirpated in B.C. are found in jurisdictions other than Washington. Both are found in Montana and Idaho, where they are either of conservation concern or unranked.
2.3.4.3 Migratory Species In B.C.
Many B.C. species migrate to areas outside the province during their life cycle. However, their migrations are often poorly understood. Many migrating species are particularly vulnerable to local threats at those times of the year when they are concentrated in large numbers or when many individuals pass through particular areas.
The taxonomic group with the largest known number of migratory species is birds. Migrants include shorebirds, waterfowl, land birds and water birds. B.C. is important as both a breeding area and migration area, with 306 species that breed in the province, but spend portions of their life elsewhere.222,223 For example, Hammond's flycatcher (Empidonax hammondii), MacGillivray's warbler (Oporornis tolmiei) and the western tanager (Piranga ludoviciana) breed in many areas of B.C., but winter in southern regions, from Baja California to Costa Rica.
Other species, such as Wilson's phalarope (Phalaropus tricolor) and Swainson's hawk (Buteo swainsoni) travel even farther, to winter in southern South America. B.C.'s location along the Pacific Flyway, which extends from Alaska to Mexico, makes it important as a wintering, migratory stopover or breeding area for many migratory birds. Significant global or continental populations of migrating shorebirds in B.C. include the black turnstone (Arenaria melanocephala), dunlin (Calidris alpina), long-billed dowitcher (Limnodromus scolopaceus), rock sandpiper (Calidris ptilocnemis), surfbird (Aphriza virgata) and western sandpiper (Calidris mauri).224 The Fraser River delta is a key area of species concentration, with up to 1.2 million sandpipers and 600,000 dunlins migrating through each year (see Section 2.5.2.1-A, p. 137),225 as is the east coast of Vancouver Island.226 Currently, approximately 25% of the North American trumpeter swan (Cygnus buccinator) population uses the Pacific Flyway to migrate to and winter on the east coast of Vancouver Island.a,227 Significant populations of geese, including 50% of the Wrangel Island population of the snow goose (Chen caerulescens), and approximately 2,000 western high-Arctic brant and black brant (Branta bernicla nigricans), use the Pacific Flyway to access key estuaries and mudflats such as those found in Boundary Bay and the Parksville-Qualicum area.228 Thayer's gulls (Larus thayeri) also breed in the Arctic and winter along the Pacific coast.229
The largest wintering population of birds of prey in Canada is found in the Fraser River delta.230 Various duck species winter along the coast, especially north of Prince Rupert, on Haida Gwaii/Queen Charlotte Islands and near estuaries in the Fraser River delta and Baynes Sound. The Interior Plateau supports the highest densities of breeding waterfowl in B.C. (approximately 420,000 breeding ducks) and serves as an important stopover point for migrating waterfowl.231 Sandhill cranes (Grus canadensis) use stopover sites in B.C. on the way to their Alaska breeding grounds, and also breed in several locations in the province.232
a Until recently, the east coast of Vancouver Island supported 50% of the continental population of the trumpeter swan. The percentage has gradually declined with the extension of this species' range into Washington State (A. Breault, Environment Canada, personal communication).
Migratory butterflies that breed in B.C. and winter in the southwestern U.S. and Mexico include the monarch (Danaus plexipMigratory butterflies that breed in B.C. and winter in the southwestern U.S. and Mexico include the monarch (Danaus plexippus), painted lady (Vanessa cardui) and west coast lady (V. annabella).233 Some bats, such as the hoary bat (Lasiurus cinereus), are thought to breed in B.C. and winter outside the province.234 The green sturgeon (Acipenser medirostris) breeds in rivers in northern California and Oregon, but migrates in small numbers to the west coast of Vancouver Island and to the Skeena River.235 Historically, this species was found in the Fraser River, but there is no evidence that it spawned in B.C. Salmon are also migratory, as they lay eggs and rear within freshwater systems and, during the marine phase of their life, many individuals migrate outside B.C. waters to feed for several years before returning to B.C. streams to spawn.
Text Box 10. Western Sandpiper: A Far-Ranging Species
The western sandpiper breeds in western Alaska and eastern Siberia, but the primary migration route for almost the entire world population of this species incorporates the B.C. coast.236 In the fall, the birds migrate south along the Pacific Flyway, pausing at major stopover sites in the Kachemak lowlands and Copper River delta in Alaska, the Stikine, Copper and Fraser River deltas in B.C., Gray's Harbor, Washington and San Francisco Bay, California, on their way to overwintering sites in the coastal southeastern U.S., northwestern Mexico and Panama Bay, and even as far south as northern Peru.237 During the northward migration back to Alaska in the spring, western sandpipers can number up to one million on a single day at Canadian and U.S. sites, aggregations that are 10 to 15 times larger than during the fall migration.
Western sandpipers use muddy intertidal habitats along their migration route, consuming seven times their body weight per day.238 Migrating sandpipers are dependent on specific stopover sites where biofilm, which accounts for 50% of their daily energy budget, is available on mud flats.239 This makes them vulnerable to changes in environmental conditions. Furthermore, this species has a relatively low rate of reproduction, making it difficult for populations to recover from impacts.
Threats, both localized and widespread, include wetland conversion and degradation, runoff of agricultural pesticides and industrial pollutants, oil spills and sea-level rise resulting from climate change, which could inundate intertidal wetlands or alter wetland ecosystems through saltwater incursion. Some threats to breeding groups, stopover sites or overwintering sites are outside B.C.'s control. The U.S. Shorebird Conservation Plan considers this to be a species of moderate concern with known or potential threats.240
2.3.5 Data Gaps
Appendix B (p.232) summarizes the state of knowledge and information gaps for the major taxa of native terrestrial and freshwater organisms in British Columbia, focusing on the availability of up-to-date species checklists, handbooks or systematic monographs, computerized geo-referenced distributional databases, and taxonomic/systematic expertise at the local (i.e., B.C.) level.
For many taxa, up-to-date species checklists are lacking and there are few handbooks or systematic monographs available.
There are computerized geo-referenced occurrence (point) databases for vertebrates, vascular plants and a few insect groups (e.g., butterflies, dragonflies and damselflies), but these are biased by being concentrated close to roads. Many parts of B.C. have never been surveyed or have not been surveyed for decades. Species distribution mapping is unavailable for all but a handful of species. These limitations affect the completeness of the species richness analyses in this report.
There is little local taxonomic expertise and many existing experts have retired and not been replaced. As elsewhere in the world, an 'extinction of experience' is occurring.241 This is particularly significant for the conservation of invertebrate and non-vascular plant species that have not yet been documented in the province or described scientifically; the number of such species is believed to be large. Except for birds, large mammals and certain salmon stocks, there is no ongoing monitoring of distribution or population trends. As a result it will be difficult to detect and respond to these changes in a timely fashion.
Conservation status has not been assessed for the majority of B.C. species, with only approximately 3,800 having been assessed out of an estimated total of 50,000,242 not including single-celled organisms. For those species that have been assessed, global status often has not been updated for over a decade.243 Information on the global range of most species is very coarse, which limits the ability to accurately estimate the proportion of the global range that occurs in B.C. Some species have been assessed in B.C., but not in neighbouring jurisdictions, making inter-jurisdictional comparisons of species status difficult.
2.4 Genetic Diversity in British Columbia
Genes are the functional units of heredity, and genetic diversity permits species to adapt to changing environments and continue to participate in life's processes (see Section 1.1, p. 5). By limiting movement between populations and creating varied ecosystems, British Columbia's complex topography, climate and glacial history have facilitated the evolution of a wide variety of local adaptations. In B.C., many species are made up of numerous geographically separate subspecies or populations, which each have a distinctive genetic makeup and characteristic appearance, environmental tolerances and/or behaviour.244,245,246,247,248,249 Recently, some of these subspecies have been shown to represent true species, that are endemic to the region,250 and it is certain that additional variation below the species level exists, particularly in taxonomic groups that have so far received little attention from science (e.g., bryophytes, invertebrates, lichens). Even in well-studied plant and animal groups, some species remain undescribed.
Many subspecies that were historically isolated in coastal B.C., Beringia or the Rocky Mountains diverged from ancestral types as glaciers advanced, then later radiated out from these historic refugia to hybridize with ancestral forms that were previously restricted to the south and east (see Section 1.4, p. 15). 251,252,253,254,255Hybrid suture zones (see Section 2.4.1.4, p. 82) can represent regions of very high genetic diversity and rapid evolution, depending on their degree of subspecies differentiation, the frequency of hybridization and the success of the hybrids produced.256
Because human activities modify natural landscapes and species distributions, humans may also influence the rate of evolution and the persistence of populations that are uniquely adapted to the B.C. environment. Although more than 60 species inhabiting B.C. have been subjects of genetic analysis,257 practical limits on research and the very large number of unstudied species generally preclude genetic classification below the species level. Consequently, biologists use simplifying concepts and measures to establish rules of thumb for conserving genetic diversity and use genetic data (where it exists) along with observations of size, shape, appearance and behaviour (collectively called phenotype) to identify distinctive lineages. Although our knowledge of genetic diversity is limited, it is vital to any discussion of biodiversity.
What Is Genetic Variation?
Genetic variation can be thought of as a species' tool kit for life, with some genes being more or less useful in current environments (adaptive genetic variation) and others having no current influence, despite being potentially influential in the future or past (neutral genetic variation). Genetically diverse populations - those with well-equipped 'toolboxes' - are thought to be best able to survive, to pass on adaptive traits to descendents and to contribute positively to their persistence. This may be particularly true for populations at the periphery of a species' range, where organisms are more likely to encounter, and potentially adapt, to novel environmental challenges, such as those associated with climate change.258,259
Genes vary in their frequency of occurrence in populations and their interaction with companion genes. Unique combinations of genes result in genetic diversity. In nature, new genes arise regularly via mutation, with beneficial, deleterious or no detectable effect on the individuals carrying them. At the population level, whether or not mutations persist depends on their effect on individuals and the size and degree of isolation of populations. Conserving genetic diversity at the population level is an overall goal of biodiversity conservation because genetic variation affects the adaptability and viability of organisms, populations and species.260,261 This is particularly important in the face of climate change.262
Genetic Variation, Divergence And Population Size
Larger populations typically retain more genetic diversity than smaller ones,263 but diversity also depends on history, population distribution and life history traits of species. The actual number of individuals in a population, referred to as the census population size, tends to overestimate genetic diversity because many factors act to reduce the variety of genes inherited by successive generations. Thus, geneticists focus on effective population size (N e): an estimate based on the number of individuals contributing genes to future generations and the rate at which populations lose genetic variation over time. Factors such as population sex ratio, mating system, population bottlenecks, growth rate, inbreeding and population fragmentation can all influence the Ne and, in doing so, affect the ability of a population to retain genetic variation.264
Genetic patterns in isolated populations are governed by the forces of mutation, drift and selection. Ne influences how genetic variation is retained in populations and, potentially, how effectively populations respond to environmental change. Ideally, natural selection removes deleterious genes and favours beneficial ones, leading to changes in gene frequency (i.e., the frequency of a gene in a given population). This process, also known as adaptation, can act rapidly in small, isolated populations, such as those on actual islands or habitat islands, and on populations at the edge of a species' range. It has undoubtedly contributed to the divergence of isolated populations inhabiting coastal archipelagos, mountain ranges, drainages and specialized habitats (e.g., karst, bogs) in B.C. For these reasons, populations that have been isolated for many generations often contain unique genetic diversity. Genetic diversity can be lost if human activities facilitate dispersal between genetically differentiated populations.
Small population size also facilitates reductions in genetic diversity and population viability, particularly in species that were once widely distributed, but have become isolated due to habitat loss and fragmentation, or whose numbers have been greatly reduced due to exploitation.265 B.C. species that have experienced severe population fragmentation and decline include the Vancouver Island marmot and many species associated with Garry oak ecosystems or inhabiting dammed rivers. Reductions in genetic diversity become more likely in such species because random effects may eliminate beneficial genes from, or embed deleterious genes in, small populations.
Overall, Ne is the single most important factor affecting genetic diversity in populations, and is therefore a key parameter in making decisions related to gene conservation. Studies of natural populations suggest that Ne averages about 11% of the census population size.266 Thus, in a population of 300 individuals, the effective population size is about 33 individuals. Long-term viability analyses indicate that minimum N e ranges from 500 to 5,000,267,268 implying census populations of 5,000 to 50,000 individuals.269
Populations or individual genotypes from one area may be genetically incompatible with local environmental conditions elsewhere. For example, in B.C., a long history of 'common garden' research trials on conifers, in which seeds from different regions are planted together, has shown that genotype can dramatically affect performance.
2.4.1 Genetic Diversity Concepts And B.C. Examples
Because genetic changes occur most rapidly in isolated populations, most studies of genetic variation in B.C. have focused on areas of historic isolation and novel environments, including islands, glacial refugia and areas at the edges of species ranges. Several areas of potential special interest are considered below, with the caveat that most B.C. taxa were scientifically described decades ago, when underlying evolutionary differences were less well known than they are currently.
2.4.1.1 Geographically Marginal Populations
There is growing evidence that geographically marginal populations (also known as peripheral populations) are often genetically different from populations at the core of the species range.270 Peripheral populations of Sitka spruce, for example, are known repositories for rare alleles (alternative forms of a gene) and locally adapted types.271 Due to B.C.'s large size and biophysical variability, many species exist as peripheral or marginal populations within the province, potentially representing evolutionarily significant lineages. Species that are at the edge of their range in B.C. may have the core of their range to the north, south or west of the province.
Distributional data is available for 3,841 B.C. taxa; of these, 2,963 species (78%) have <10% of their range, area or population within the province (see Table 16, p. 61).272 Some of these geographically marginal populations show clearly defined genetic variation and are of conservation concern. They include several species confined to the South Okanagan-Similkameen region (e.g., sage thrasher, Mormon metalmark [Apodemia mormo], Behr's hairstreak [Satyrium behrii]) and the Gulf Islands (e.g., propertius duskywing [Erynnis propertius], Edith's checkerspot [Euphydryas editha taylori]). Peripheral species or marginal populations in northern B.C. include two butterflies: the eastern pine elfin (Callophrys niphon), which is confined to the northeast provincially, and the phoebus parnassian (Parnassius phoebus), which is found in Siberia, Alaska and the western Yukon, as well as the northwestern corner of B.C.273
2.4.1.2 Island And Disjunct Populations
In many taxa, island populations are recognized as subspecies due to phenotypic differences and geographic isolation. Similarly, disjunct populations, which are isolated either by a geographical or environmental barrier within a former contiguous range or by long-distance dispersal, can be subspecies.274 Many island and disjunct subspecies are endemic to B.C. and a number of them are of conservation concern (Table 20).
The Kermode or spirit bear is an impressive example of the insular effect on genetically based traits (see Figure 3, p. 7). The trait known as kermodism is expressed as a white coat displayed by any bear that carries two copies of a certain recessive allele. Genetic analyses indicate that most coastal black bears, regardless of colour, have descended from populations that were once restricted to glacial refugia and were the source of the recessive allele; those populations now mix with continental lineages, which lack this trait.275 However, the white individuals are most common on islands. The high frequency of this trait on coastal islands (perhaps 25% of individuals in some populations) is consistent with the idea that water-barriers to dispersal and small population size have acted to increase its frequency via random genetic drift.276
Table 20. B.C. Endemic Taxa Below The Species Level That Are Of Provincial Conservation Concern.
| Scientific Name | Common Name | Provincial Conservation Status |
| Birds | ||
| Aegolius acadicus brooksi | Northern saw-whet owl, brooksi subspecies | Imperilled |
| Cyanocitta stelleri carlottae | Steller's jay, carlottae subspecies | Vulnerable |
| Glaucidium gnoma swarthi | Northern pygmy-owl, swarthi subspecies | Vulnerable |
| Lagopus leucura saxatilis | White-tailed ptarmigan, saxatilis subspecies | Vulnerable |
| Picoides villosus picoideus | Hairy woodpecker, picoideus subspecies | Vulnerable |
| Pinicola enucleator carlottae | Pine grosbeak, carlottae subspecies | Vulnerable (breeding pop) |
| Freshwater Fish | ||
| Acipenser transmontanus pop. 3 | White sturgeon (Nechako River population) | Critically imperilled |
| Acipenser transmontanus pop. 4 | White sturgeon (Lower Fraser River population) | Imperilled |
| Acipenser transmontanus pop. 6 | White sturgeon (Middle Fraser River population) | Critically imperilled |
| Coregonus sp. 1 | Dragon Lake limnetic whitefish | Extinct |
| Coregonus sp. 1 | Dragon Lake benthic whitefish | Extinct |
| Cottus sp. 2 | Cultus pygmy sculpin | Critically imperilled |
| Gasterosteus aculeatus pop. 1 | Charlotte unarmoured stickleback | Imperilled |
| Gasterosteus sp. 1 | Giant black stickleback | Critically imperilled |
| Gasterosteus sp. 12 | Hadley Lake limnetic stickleback | Extinct |
| Gasterosteus sp. 13 | Hadley Lake benthic stickleback | Extinct |
| Gasterosteus sp. 16 | Vananda Creek limnetic stickleback | Critically imperilled |
| Gasterosteus sp. 17 | Vananda Creek benthic stickleback | Critically imperilled |
| Gasterosteus sp. 18 | Misty Lake "lake" stickleback | Critically imperilled |
| Gasterosteus sp. 19 | Misty Lake "stream" stickleback | Critically imperilled |
| Gasterosteus sp. 2 | Enos Lake limnetic stickleback | Critically imperilled |
| Gasterosteus sp. 3 | Enos Lake benthic stickleback | Critically imperilled |
| Gasterosteus sp. 4 | Paxton Lake limnetic stickleback | Critically imperilled |
| Gasterosteus sp. 5 | Paxton Lake benthic stickleback | Critically imperilled |
| Lampetra richardsoni pop. 1 | Western brook lamprey (Morrison Creek population) | Critically imperilled |
| Spirinchus sp. 1 | Pygmy longfin smelt | Critically imperilled |
| Thymallus arcticus pop. 1 | Arctic grayling (Williston Watershed population) | Critically imperilled |
| Mammals | ||
| Gulo gulo vancouverensis | Wolverine, vancouverensis subspecies | Possibly extinct |
| Microtus townsendii cowani | Townsend's vole, cowani subspecies | Critically imperilled |
| Mustela erminea anguinae | Ermine, anguinae subspecies | Vulnerable |
| Mustela erminea haidarum | Ermine, haidarum subspecies | Imperilled |
| Neotamias minimus selkirki | Least chipmunk, selkirki subspecies | Critically imperilled |
| Sorex palustris brooksi | American water shrew, brooks subspecies | Imperilled |
| Butterflies | ||
| Plebejus saepiolus insulanus | Greenish blue, insulanus subspecies | Possibly extinct |
| Vascular plants | ||
| Viola biflora ssp. carlottae | Queen Charlotte twinflower violet | Vulnerable |
| Lloydia serotina var. flava | Alp lily | Vulnerable |
| Trillium ovatum var. hibbersonii | Dwarf trillium | Critically imperilled |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Both Haida Gwaii/Queen Charlotte Islands and Vancouver Island are home to a wide array of subspecies. In northern B.C., Hecate Strait has been a formidable barrier to dispersal, contributing to the distinctiveness of several bird and mammal species on Haida Gwaii/Queen Charlotte Islands and adding to the historic importance of these islands as a glacial refugium (see Section 2.4.1.3, p. 78). Dawson caribou (Rangifer tarandus dawsoni) historically inhabited Haida Gwaii/Queen Charlotte Islands, but this small forest caribou subspecies was last seen in 1908.277 Other mammal subspecies unique to the archipelago include the largest subspecies of black bear (Ursus americanus carlottae) and a subspecies of ermine (Mustela erminea haidarum) that was once relatively common, but is now thought to be extinct or reduced to very low numbers. Notable birds found on these islands include subspecies of the Steller's jay (Cyanocitta stelleri carlottae), hairy woodpecker (Picides villosus picoideus), pine grosbeak (Pinicola enucleator carlottae) and northern saw-whet owl (Aegolius acadicus brooksi).
Endemic species and subspecies found on Vancouver Island include the critically imperilled Vancouver Island marmot, the Vancouver Island wolverine (Gulo gulo vancouverensis), which has not been seen since 1982, a white-tailed ptarmigan subspecies (Lagopus leucura saxatilis) and a northern pygmy-owl subspecies (Glaucidium gnoma swarthi). A number of butterfly subspecies and endemic plants are also found on Vancouver Island.
Although genetic comparisons of species in this region remain scarce, recent studies of the northwestern and North American deermouse (Peromyscus keenii and P. maniculatus, respectively), both resident in coastal B.C., suggest that glacial history and small effective population sizes have led to substantial genetic differentiation between populations. This raises the possibility that additional taxa of B.C. plants and animals remain undescribed, particularly on coastal islands and within many sedentary species of plants, vertebrates, and invertebrates (Text box 11).
2.4.1.3 Glacial Refugia
Haida Gwaii/Queen Charlotte Islands and the Brooks Peninsula on Vancouver Island, two of the most prominent areas identified as glacial refugia within B.C., provide homes to a significant component of the province's genetic biodiversity. For example, Haida Gwaii/Queen Charlotte Islands, which encompasses 250 islands, has been termed 'the Galapagos of the North' due to the archipelago's high levels of biodiversity and relict species, including numerous endemic species: five vascular plant species, four insects, two liverworts (hepatics) and five mosses. However, the isolation and ecological novelty that gave rise to such diversity also makes these areas vulnerable, and both areas have been significantly impacted by alien species. Since Sitka black-tailed deer were introduced to Haida Gwaii/Queen Charlotte Islands in the late 1800s, they have dramatically altered the ecology of entire rainforest ecosystems, with deleterious impacts on many species (see Section 1.1.2, p. 7).281 In addition to introducing alien species, human activities have the potential to disrupt island populations by reducing historic barriers to dispersal between divergent populations, and converting and fragmenting significant habitats. When activities lead to demographic decline or to the dilution or loss of locally adapted traits, extinction risk increases. Much of the species-level diversity evident in B.C. freshwater fish is a product of Pleistocene range fragmentation and genetic divergence, followed by recolonization from refugia.282 For example, the Salish sucker (Catostomus sp. 4) and Nooksack dace (Rhinichthys sp. 4) may be the only Canadian representatives from the
Text Box 11. Cryptic Species: Diversity Hiding In Plain Sight
The advent of genetic analysis has begun to reveal a large number of 'cryptic' species. These are cases where what was previously considered to be a single species is found to actually be a complex of two, and sometimes more, species that are very similar in appearance. Species do not have to be small to be cryptic. Recently, the African elephant was recognized as two genetically distinct, non-interbreeding species, one retaining the name African elephant (Loxodonta africana) and the other now known as the African forest elephant (Loxodonta cyclotis).278 In B.C., the seaside juniper (Juniperus maritima) was recently described based on genetic and other information; it was previously included in the Rocky Mountain juniper (Juniperus scopulorum).279 Another example of this hidden diversity is the possible division of the winter wren (Troglodytes troglodytes) into two species with a contact zone in northeastern B.C.280
Further genetic analysis is very likely to identify more cryptic species and thereby add to the number of recognized species in B.C.
Chehalis refugium, which was centred around southern Puget Sound during the most recent glacial maximum283 (Text box 12), although a recent study of the Olympic shrew (Sorex rohweri)a indicates colonization from the same refugium.284 The Salish sucker has no formal taxonomic status, but is identified as an evolutionarily significant unit. Similarly, the Nooksack dace has not been given a formal taxonomic rank, since it is not yet clear whether it is a true species or is a subspecies of the widespread longnose dace (Rhinichthys cataractae).285 Both the Nooksack dace and the Salish sucker are of conservation concern. Another example is the pygmy whitefish (Prosopium coulterii). While scattered populations of pygmy whitefish are found across northern North America, usually in deep, nutrient-poor lakes, two nutrient-rich B.C. lakes are home to a 'giant' form that is found nowhere else.
Although glacial retreat restored connectivity between many populations of plants and animals, it isolated others as the land rebounded from under the immense weight of the ice. The rising land mass confined some anadromous fish, such as the Pacific lamprey (Lampetra tridentata) and longfin smelt (Spirinchus thaleichthys), to freshwater locations, resulting in rapid divergence of new forms. In some cases, this process produced species endemic to British Columbia. A particularly well-researched example involves the complex genetics of sticklebacks in six lakes on three islands in the Strait of Georgia. Each lake has given rise to two forms of sticklebacks: benthics, which are stout and wide-mouthed and forage at lake margins; and limnetics, which are slender and slim-mouthed and forage in the open waters of the lake. The two forms carry different alleles and rarely hybridized until recent human influences altered these communities. The genetic differences evident in these species are particularly interesting because they appear to have arisen very recently (since the last ice age) from a common ancestor and in parallel in all three lakes. Because these differences represent adaptive genetic variation that affects individual survival and reproduction and, therefore, population persistence, the forms are each recognized as endemic taxa.286 Such patterns of divergence provide a remarkable snapshot of evolution in action.
Recent DNA studies of the alpine plant, mountain sorrel, suggest the existence of one or more refugia in the mountains of northern B.C.287 Other genetic research shows that southern red-backed voles (Myodes gapperi) at higher altitudes are more closely related to central continental populations than to eastern or western populations.288
a There has not been an official publication on the common name. An alternative possibility is Rohwer's shrew.
Text Box 12. Fish And Glacial Refugia289
From the four corners of the province, more than 65 fish species recolonized B.C. after the last glaciation, originating from three major refugia: the Pacific (including the Chehalis and Columbia minor refugia), the Great Plains, and the Beringian (including the Nahanni) (Figure 20) . Twenty-four of these species (36%) came from more than one unglaciated area. Therefore, much of the province's within-species diversity in fish is due to range fragmentation as a result of glaciation, followed by genetic divergence and subsequent recolonization from different refugia.
The Pacific refugium was the largest contributor to B.C. fish fauna, with fish using two dispersal routes: salt water and fresh water. Species that dispersed by sea included lampreys (Lampetra spp.), sturgeons (Acipenser spp.), smelts (Spirinchus spp.), trout and salmon (Oncorhynchus spp.) and sticklebacks. Those that were restricted to freshwater habitats and required drainage connections included northern pikeminnow (Ptychocheilus oregonensis) and suckers (Catostomus spp.). Species that could tolerate harsher conditions were able to move from the Columbia refugium to northern areas such as the Nass, Skeena and Peace river drainages, while species unable to tolerate those conditions were able to colonize only the Fraser River system.
One of the minor refugia, the Chehalis, provided a dispersal route from Puget Sound in Washington, allowing two saltwater-intolerant fish, the Salish sucker and Nooksack dace, to disperse into the lower Fraser River valley.
With the retreat of the glaciers, fish species migrated through the Columbia system into several large glacial lakes (including Kamloops, Thompson and Shuswap lakes), allowing species such as the peamouth (Mylocheilus caurinus), longnose dace and northern pikeminnow to disperse farther north. Oncorhynchus fossils found near Savona in Kamloops have been dated at 18,000 years ago,290 indicating that salmon existed in the province 3,000 to 4,000 years before the glacial maximum. The connection between these lakes and the Columbia River was later severed and the lakes then drained into the Fraser River system,291 eliminating the opportunity for other species from the Columbia River to colonize farther north. In fact, coho salmon that now occur in the interior reaches of the Fraser River came from the Columbia River basin292 and have sufficient genetic differences to distinguish them from coho salmon in the lower Fraser River.293 Thirteen thousand years ago, a corridor between the Missouri River system and the lower Peace River294 allowed species from the Great Plains refugium, such as goldeye (Hiodon alosoides) and flathead chub (Platygobio gracilis), to colonize northeastern B.C. After this period, intermittent connections with other drainages, such as the Fraser River drainage, allowed colonization by some other Great Plains species (e.g., white sucker [Catostomus commersonii], lake whitefish [Coregonus clupeaformis] and brassy minnow [Hybognathus hankinsoni]). Another temporary connection to northeastern B.C. occurred from the Mississippi River system through the Canadian prairies (Lake Agassiz in Saskatchewan and Manitoba), and from the Northwest Territories to the Beaufort Sea. Although the Nahanni refugium in northeastern B.C. is not accepted by all researchers,295 there is evidence that it was a source of colonizing lake whitefish296 and lake trout (Salvelinus namaycush).297 Compared to the Pacific and Great Plains refugia, the Beringian refugium contributed a relatively small number of species. There were some limited opportunities for species to disperse to the Liard and Stikine river systems from the Yukon and portions of Alaska. Species that were able to colonize during this short period include the round whitefish (Prosopium cylindraceum) and Arctic grayling (Thymallus arcticus).298 Beringian refugium species that were able to use marine environments, such as salmon, colonized southward, while salmon from the Pacific refugium moved northward.299
Figure 20: The three major ice-free refugia from which freshwater fish recolonized British Columbia.
Source: McPhail, J.D. 2007. Freshwater Fishes of British Columbia. University of Alberta Press, Edmonton, AB. 620pp.
2.4.1.4 Major Hybridization Zones
Hybrids often hold a tenuous place in conservation because, once they are detected, the appropriateness of the 'species' designation for the two forms involved is often questioned. Although hybridization is a potentially serious problem for populations with unique evolutionary histories, many naturally occurring hybrid zones are known to be stable in ecological timeframes, perhaps contributing novel lineages and species in evolutionary time. For plants in particular, hybridization is an important process for creating new species300,301 Hybrid zones are therefore fascinating laboratories for evolutionary study and potential hot spots of genetic variation and local adaptation.
Research suggests that in North America there are 13 hybrid suture zones,302 where divergent taxonomic groups overlap and some species hybridize as a result of landscape change and the historic expansion and contraction of species ranges. Although not well studied, B.C. likely has the highest density of these zones in Canada. One major suture zone extends from the southeast corner of the province to the central interior, representing the channelling effects of mountain ranges on species as they radiated across the landscape during global shifts in climate.303 Several 'superspecies' (complexes of closely related species) are found in this region; many of them rarely hybridize, but some do so extensively. For example, the northern flicker (Colaptes auratus) occurs across North America, but in a band stretching from B.C. to Texas, 95% of the flickers are hybrids between the red-shafted and yellow-shafted subspecies or between the red-shafted subspecies and the closely related gilded flicker (Colaptes chrysoides).304
Another species that provides insights into the role of continental divides in divergence and speciation is Swainson's thrush (Catharus ustulatus), which has two distinct populations, one coastal and one continental, with both populations found in B.C.305 A similar east-west separation occurs amongst other populations of land birds, such as Wilson's warbler (Wilsonia pusilla).306 The Okanagan and Kootenay valleys are the only place in North America where two species of tiger swallowtails (Papilio spp.) have overlapping ranges and are known to hybridize.307 For freshwater fish, a major hybridization zone exists in the lower Peace River system, which provided a postglacial dispersal corridor where several western populations crossed and hybridized with eastern populations.308
2.4.2 Status Of British Columbia Taxa Below The Species Level
Only about 60 B.C. taxa have been the subject of peer-reviewed genetic studies and those studies have focused mainly on evolutionary history, population genetic structure, geography of evolutionary lineages and fine-scale effects of forest practices on genetics and hybridization.309 Eight of the studies (four on fish, two on birds and one each on a mammal and an invertebrate) recognized evolutionarily significant units below the species level.
Table 21 summarizes the conservation status of 457 B.C. taxa below species level and shows the number that have the majority of their global range in B.C. However, it provides only a limited picture of the status of genetic diversity in the province, as a reliable list of taxa below the species level is not available for most groups and, of those that are known, many have not been assessed. The lack of described taxa below the species level does not necessarily indicate a lack of genetic differentiation at that level. Conservation status rankings are explained in Section 2.3.2 (p. 51) and proportion of global range is explained in Section 2.3.3 (p. 61).
Eleven subspecies or populations have been assessed as extinct or extirpated (Table 22), including three birds, four freshwater fish, two mammals, one reptile and one butterfly.
Table 21. Number Of Taxa Below The Species Level Of Global And Provincial Conservation Concern, As Well As Those That Have A Majority Of Their Global Range In B.C.
| Taxonomic Group | Number Of Taxa Of Global Conservation Concern | Number Of Taxa Of Provincial Convervation Concern | Number Of Taxa With Majority Of Global Range, Distribution Of Population In B.C. |
| Vertebrates | |||
| Amphibians | 0 | 0 | 0 |
| Birds | 0 | 24 | 8 |
| Freshwater Fish | 11 | 29 | 25 |
| Mammals (Excluding Cetaceans) | 0 | 20 | 6 |
| Reptiles And Turtles | 0 | 4 | 0 |
| Invertebrates | |||
| Butterflies And Skippers | 1 | 32 | 8 |
| Dragonflies And Damselflies | 0 | 0 | 0 |
| Non-Marine Molluscs | 0 | 1 | 0 |
| Coleopterans (Beetles) | 0 | 0 | 0 |
| Vascular Plants | |||
| Ferns And Fern Allies | 1 | 8 | 0 |
| Conifers | 0 | 0 | 0 |
| Monocots | 0 | 43 | 3 |
| Dicots | 2 | 220 | 16 |
| Non-Vascular Plants | |||
| Mosses | 0 | 76 | Not Assessed |
| Total | 15 | 457 | 66 |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Notes: Taxa below the species level include subspecies, populations and varieties, as well as taxa lacking formal scientific species names (e.g., Gasterosterus sp.1). Some taxa that are of provincial conservation concern (e.g., the mountain caribou ecotype) are not assessed globally. In cases where only one taxon below the species level occurs in B.C., it has been included in the species analysis and not included here.
2.4.3 Data Gaps
Relatively little is known about the status of genetic diversity in B.C., particularly for certain taxonomic groups. While some species of fish and birds have been the subjects of multiple studies, genetic data are rare to nonexistent for amphibians, invertebrates, bryophytes and vascular plants other than trees. The existing designation of subspecies is questionable in some cases and there is disparity in how biologists deal with variation in different taxonomic groups (i.e., subspecific taxa are not formally described in all groups). The fact that molecular markers for given species can often be readily applied to close relatives means that genetic surveys will become more feasible in future. There are important data gaps in relation to appropriate taxonomic units for subspecies that are difficult to differentiate (see Text box 11, p.78) and populations of particularly high or low genetic diversity.
Identifying uniquely adapted taxa documents genetic diversity and divergence, and may contribute to the persistence of local populations. Hot spots of genetic distinctiveness 310 and divergent populations may be identified by observable physical characteristics in some cases, but some taxa are difficult to differentiate and require genetic markers for identification.311
Table 22. Extinct And Extirpated Taxa Below The Species Level In B.C.
| Taxonomic Group | Scientific Name | Common Name | Conservation Status |
| Birds | Eremophila Alpestris Strigata | Horned Lark, Strigata Subspecies | Extirpated |
| Melanerpes Lewis Pop. 1 | Lewis's Woodpecker | Extinct (Georgia Depression Population) | |
| Sturnella Neglecta Pop. 1 | Western Meadowlark | Extirpated (Georgia Depression Population) | |
| Freshwater Fish | Coregonus Sp. | Dragon Lake Limnetic Whitefish | Extinct |
| Coregonus Sp. | Dragon Lake Benthic Whitefish | Extinct | |
| Gasterosteus Sp. 12 | Hadley Lake Limnetic Stickleback | Extinct | |
| Gasterosteus Sp. 13 | Hadley Lake Benthic Stickleback | Extinct | |
| Mammals | Bos Bison Bison | Plains Bison | Extirpated |
| Rangifer Tarandus Dawsoni | Dawson Caribou | Extinct | |
| Reptiles And Turtles | Pituophis Catenifer | Catenifer Gopher Snake, Catenifer Subspecies | Extirpated |
| Butterflies | Euchloe Ausonides Insulanus | Island Large Marble | Extirpated |
Source: Prepared for this report with data from the B.C. Conservation Data Centre.
Notes: The only plains bison currently found in B.C. are considered aliens because they belong to an introduced population located outside the historic range of this subspecies. Kincaid's lupine (var. kincaidii) is listed at the species level in Table 14 (p. 58) because it is the only representative of Lupinus oreganus that occurs in B.C.
Text Box 13. Mountain Caribou In Southeastern British Columbia
The woodland caribou (Rangifer tarandus caribou) is one of four subspecies of caribou currently found in North America and the only one occurring in British Columbia.a There are three ecotypes of woodland caribou: boreal, northern and mountain. Of these, the mountain caribou ecotype is of the greatest conservation concern, due to its small population size.
Mountain caribou populations have probably existed in southern B.C. for more than 10,000 years and likely advanced and retreated with glacial events.312 Mountain caribou are found primarily in the Cariboo, Selkirk, Purcell, Monashee and Rocky mountains in the southeastern part of the province (Figure 21). They are threatened by ecosystem degradation, including fragmentation, disturbance, predation (due to altered predator-prey dynamics resulting from ecosystem degradation) and climate change.313,314 The estimated population of mountain caribou has decreased by 17% from 2,300 in 2000, to about 1,900 in 2006.315 These animals constitute almost all of the global population of this ecotype.316 Since 2000, two of the 18 herds - the George Mountain and Central Purcells herds - have been extirpated. Extirpation of a herd results in the loss of that population's genetic contribution to the larger population.
In 2007, the provincial government announced the Mountain Caribou Recovery Implementation Plan, with the goal of restoring the mountain caribou population to pre-1995 levels of more than 2,500 animals throughout their existing range. The plan includes: habitat conservation; managing human recreational activities; managing wolf and cougar populations where they threaten caribou recovery; managing the prey of mountain caribou predators; augmenting small herds by transplanting caribou; and adaptive management, including monitoring.317
a A fifth subspecies, the Dawson caribou, is extinct (see Table 22, p. 84).
Figure 21: Distribution of the three ecotypes of woodland caribou in B.C.
Source: B.C. Integrated Land Management Bureau.
Genetic analyses can provide information about how historic and recent population-size fluctuations have affected population viability, migration routes, dispersal barriers and sex ratios.318 For example, uncertainty about the value of dispersal corridors for a particular species might be resolved by genetic analysis to identify whether historic dispersal patterns have been interrupted by habitat fragmentation and whether re-established corridors are actually used.319
Data gaps for specific taxonomic groups are summarized below.
Mammals: Mammals are one of the best-studied groups, but most taxonomic studies rely on physical features and have not been confirmed by genetic analysis. Genetic studies using molecular markers for closely related species (e.g., bats) are lacking. Another gap is identification of subspecies that are difficult to differentiate, especially for taxonomic groups in historic refugia and geographically disjunct populations that exhibit low dispersal ability despite having a moderate to large effective population size.
Birds: There is an established history of genetic research on birds. In B.C., several studies of birds are currently being undertaken to investigate how hybrid suture zones contribute to biodiversity through both speciation and hybridization320 and how historic refugia and population isolation affect micro-geographic variation in phenotype, genetic diversity and population persistence.321
Freshwater fish: Freshwater fish populations exhibit high genetic differentiation322 and often differ in phenotype and genotype across major drainages in B.C. due to historic and recent isolation.323 For example, there are more than 400 genetically distinct populations among five species of Pacific salmon in B.C.324 Because of a long history of commercial, recreational and scientific interest, this group is among the best studied with respect to taxonomic and genetic diversity. However, genetic studies that use recent taxonomic reviews are lacking.
Amphibians: This group may incorporate higher levels of genetic differentiation in B.C. than are currently recognized, since meta-analysis (a method of analysis that combines the results of a number of studies to investigate underlying processes) shows that amphibian populations tend to be more differentiated than bird populations325 and since some amphibians potentially have isolation histories similar to those of fish. Genetic research that considers geographic distribution and the potential for adaptive divergence based on life history is lacking.
Reptiles and turtles: Information about divergence in geographically isolated populations is lacking. Many detailed studies of genetic differentiation in adaptive traits related to predation and colouration have focused on the garter snakes (Thamnophis spp.), including species common in B.C. and on coastal islands.
Invertebrates: Genetic studies of invertebrates elsewhere in the world often find strong differentiation within species based on geographic distribution and food-plant specialization. This suggests that there may be genetic variants in B.C. that have not been described. One study of ground beetles (Nebria charlotte and N. haida) on Haida Gwaii/Queen Charlotte Islands indicates genetic variability within that population.326 No peer-reviewed genetic studies of dragonflies, damselflies or non-marine molluscs have been done in B.C.
Vascular plants: The degree to which differences between populations are due to different environmental conditions (e.g., soil nutrients) or genetic differences stemming from adaptation to historically isolated sites is not well understood. A long history of empirical studies has demonstrated pronounced adaptive divergence among conifer populations in B.C. More recently, this has been also demonstrated for some Garry oak ecosystem plants, including sea blush (Plectritis congesta), broad-leaved stonecrop (Sedum spathulifolium)327 and blue-eyed Mary (Collinsia spp.).328
Non-vascular plants: No peer-reviewed genetic studies of these taxa in B.C. have been published.
Text Box 14. The Hidden Majority329
Many elements of biological diversity are overlooked because they are either too small to see easily or are processes rather than single plants or animals. For example, many invertebrates are hidden in soil or under the bark of trees or are microscopic. An estimated two million soil invertebrates inhabit every square metre of ground in the Pacific Northwest.330 About 30 to 40 species of oribatid mites, totalling 40,000 to 50,000 individuals, live on the surface of a single stump, feeding on 25 or so species of fungi.331,332,333 There are at least 94 species of terrestrial snails and slugs in B.C.334
Invertebrates make up most of the 'hidden majority' of species, but they are only part of B.C.'s little-noticed diversity (Figure 22). Forty to 75 species of mosses, liverworts and lichens can grow on the trees in a forest plot the size of a football field (approximately 0.5 ha),335,336,337,338,339,340 with a mass of up to 2.6 tonnes per hectare in coastal old-growth forests.341 An additional 20 to 50 species of crustose lichens342 and many more bryophytes live on the rocks and soil.
Even though they are not easily identified by most people, these small organisms perform critical functions such as nutrient cycling. For example, mosses absorb up to 10 times their weight in water and help regulate water flow (see Section 2.5.1.3-B, p. 115). The role played by many species in ecosystem functioning is unknown, and the inadvertent loss of critical species is a very real danger.
Figure 22: The hidden majority plays a weighty role in the functioning of ecosystems.

Source: F. Bunnell and I. Houde, University of British Columbia. illustration: I. Houde.
2.5 Selected Key and Special Elements of Biodiversity in British Columbia
Genes, species, ecosystems and the processes that link them are the major components of biodiversity, but not all are equal when it comes to conserving other elements of biodiversity.
Section 2.5.1 examines key elements - pieces of the biodiversity puzzle that are essential and/or have a disproportionate influence on ecosystem function. Many of these key elements are not tidily encompassed by the three levels of organization (genes, species and ecosystems). Section 2.5.2 (p.137) looks at special elements - elements of biodiversity that are uncommon and often globally significant.
2.5.1 Key Elements
The following pages describe a small subset of the multitude of components, structures and functions of biodiversity that help sustain life on earth. These key elements are known to play fundamental or disproportionately large roles in the functioning of ecosystems. For each element, its status in B.C. is described, if known.
Table 23 lists the key elements chosen to illustrate some of the functions, structures and components that are essential for maintaining healthy ecosystems and the services derived from them. These were chosen at a series of workshops in 2007, using a framework to identify essential ecosystem characteristics.343 Each composite piece is engaged in functions and creates structure. The more that communities of species remain intact, the more likely it is that ecological processes will be maintained. Scientific knowledge is generally insufficient to evaluate the apparent importance of the processes or the species involved (except in the case of a few well-documented keystone species). With some exceptions, the value of many elements of biodiversity is unknown until they fail.
2.5.1.1 Cross-Realm Elements
A. Connectivity
What is it? Connectivity is the degree to which ecosystem structure facilitates or impedes the movement of organisms between resource patches.344 What constitutes connectivity is scale-dependent and varies for each species depending on its habitat requirements, sensitivity to disturbance and vulnerability to human-caused mortality.345 A spotted owl (Strix occidentalis) may avoid flying across large clearcuts,346 while a grizzly bear may avoid crossing a highway with a high volume of traffic.347 A smaller organism such as a butterfly, which may fly no more than a few hundred metres in its lifetime, can be dramatically affected by urban or agricultural development.348 In stream systems, connectivity occurs upstream and downstream (see Section 2.5.1.3-D, p. 118), between groundwater and surface water (see Section 2.5.1.3-E, p. 119), and between aquatic and terrestrial ecosystems.
Why is it important? Connectivity allows individual organisms to move in response to changing conditions, such as seasonal cycles, a forest fire or climate change. It also permits linkages between individuals in geographically separated populations. Connected populations are much less vulnerable to being extirpated as a result of chance or random events, because they can be 'rescued' or recolonized by immigration from other populations.349 In addition, large, connected populations are more influenced at a genetic level by natural selection, while small, fragmented populations are vulnerable to the random, often damaging effects of genetic drift and inbreeding.350 Freshwater species that are confined to water cannot escape the deleterious effects of lost connectivity. Poorly designed stream crossings can disrupt connectivity by preventing fish passage upstream or downstream. This can prevent the dispersal of some aquatic invertebrates, such as freshwater mussels,a whose larvae travel throughout stream systems on the fins or gills of fish.351 Freshwater mussels perform important ecological functions such as water filtration.
Status/threats in B.C. For many species in both the terrestrial and freshwater realms, ecosystem conversion and degradation are resulting in the loss of connectivity (also referred to as habitat fragmentation). Species that rely on the core areas of old-growth forests are less able to move through landscapes where large areas have been recently logged. Sensitive species such as grizzly bears, wolverines (Gulo gulo) and bull trout
(Salvelinus confluentus) avoid areas with high levels of human use.352 Off-road use of four-wheel drives, ATVs and snowmobiles can also contribute to the loss of connectivity for sensitive species such as elk (Cervus canadensis)353 and mountain caribou354 (see Section 3.3.8, p. 205).
a There are six species of freshwater mussels in B.C. The rarest is the Rocky Mountain ridged mussel (Gonidea angulata), found only in the Okanagan and Kootenay rivers.
An estimated 66,000 stream crossings were built in B.C. between 2000 and 2005, with an average increase of 13,369 stream crossings per year.355,356 A 2006 assessment of 178 culverts in the Carp and MacGregor river watersheds found that 88% of the culverts were a potential barrier to fish passage.357 Figure 23 shows the amount of potential fish habitat that is lost upstream of a culvert that fails to allow fish passage.
Figure 23: Potential loss of fish habitat owing to stream crossings that block fish passage.
Illustration: Soren Henrich.
Data gaps: Rigorous assessments to identify linkages have not been conducted for most of the province and much of the work that has been done has focused on large carnivores.358,359,360,361 There is also the issue (which is not unique to B.C.) of determining exactly what constitutes linkage for particular species.
B. Riparian Areas
What are they? Riparian refers to the transition zone between an aquatic and a terrestrial system that is influenced by either surface or subsurface water. A riparian area may be located beside a lake or estuary or an ephemeral, intermittent or perennial stream or creek. Riparian areas are dynamic ecosystems that may be subject to temporary, frequent or seasonal flooding. They support plant communities that tolerate moister conditions than those found in upland areas. They are typically linear, but can extend over large landscapes and are found in all areas of the province.
Hydro-riparian ecosystems extend beyond the riparian zone to encompass both the water and the adjacent land in one integrated ecosystem that includes aboveand below-ground processes.362,363,364
Why are they important? Riparian area functions include the influence of land on adjacent water, the influence of water on adjacent land, and connectivity.365 Land influences adjacent water as vegetation moderates temperature and water input, filters sediment, provides structure and nutrients and stabilizes banks. In addition, bedrock and soil determine water chemistry and channel form. Water influences adjacent land by eroding banks, depositing sediments that create soil, modifying microclimates and influencing vegetation and productivity.
In well-drained soils, flooding creates mosaics of diverse and productive communities. Riparian and hydroriparian ecosystems link landscapes, providing corridors for animal and plant movement, sediment transport and water transport. In hydro-riparian ecosystems, the connectivity includes underground connections (see Section 2.5.1.3-E, p. 119). The phreatic (groundwater) zone and the hyporheic zone (the saturated sediment zone between groundwater and surface waters) provide water purification and transport, as well as habitat and nutrients for plants and animals.
Riparian wetlands play an important role in storage and filtering of water, the maintenance of water quality and the reduction of sediment levels, nutrients and toxic chemicals in outflow water.
Riparian areas support a variety of vegetation cover types, from trees and shrubs to emergent and herbaceous plants. Because of this high plant diversity, they provide foraging, nesting and/or breeding habitat for an abundance of terrestrial and freshwater life (Figure 24).366,367,368 The vegetation in riparian areas directly influences and provides important fish habitat.369 It builds and stabilizes stream banks and channels, provides cool water through shade370 and provides shelter for fish. The leaves and insects that fall into the water are a source of food for fish.371,372 The hyporheic zone in hydro-riparian areas provides habitat for a variety of insects and microfauna, which are in turn prey for larger species. It is a source of nutrients and water for plants and is where much of the water purification occurs, reducing sediment levels, nutrients and toxic chemicals in outflow water. Accounting for only a small portion of British Columbia's land base, riparian areas are often more productive than the adjoining upland. Hydro-riparian ecosystems are important as hot spots of biodiversity.373
Figure 24: Relationships between riparian areas and terrestrial and freshwater species.
Source: Adapted from B.C. Ministry of Water, Land and Air Protection. 2006. Riparian Areas Regulation Implementation Guidebook. Biodiversity Branch, Victoria, BC. 87pp. Available at: www.env.gov.bc.ca/habitat/fish_protection_act/riparian/documents/ImplementationGuidebook.pdf. illustration: Soren Henrich.
Although riparian areas generally support disproportionately high numbers of species relative to the area they occupy on the land base, their presence is even more critical in dry ecosystems such as grasslands. Riparian wetlands adjacent to grasslands support a variety of species of conservation concern. They contain high-quality habitat that provides for many of the diverse needs of species, including water, food and cover. In areas where grasslands dominate, lowland riparian areas may be the only source of large trees and snags for several kilometres, providing habitat for species that would otherwise not be present. For example, although Lewis's woodpeckers (Melanerpes lewis) are adapted to recent fire-created ecosystems, in B.C. the highest nesting density of this species of conservation concern is in lowland riparian areas in the Okanagan Valley - a higher density than in coniferous forests in the same area.374,375,376 The macfarlanei subspecies of the western screech owl (Megascops kennicottii macfarlanei) is very closely associated with riparian habitats below 950 m and this dependence is at least partly related to the presence of cottonwoods of sufficient size to provide nest cavities.377
Status/threats in B.C. Riparian systems are affected by topography, surficial materials and the duration and magnitude of flood events. These factors influence their response to disturbances. Intensive recreational activities along the edges of wetlands can reduce plant cover, compact soil and disturb nesting birds. However, riparian wetlands are known to be resilient in response to disturbance.378 Tree mortality caused by mountain pine beetles also affects riparian areas (see Text box 16, p. 105).
Riparian areas and adjacent wetlands are rare in grassland landscapes. They are extremely vulnerable to vegetation removal, filling or draining, and overuse by livestock.379,380 Riparian areas in dry ecosystems are often lost through conversion of stream banks and riparian wetlands to fields, lawns and pavement owing to urbanization and agriculture (see Text box 5, p. 39).
In the Okanagan region, 63% of the black cottonwood /water birch (Betula occidentalis) riparian shrub wetland ecological community and 92% of the water birch /roses (Rosa spp.) riparian shrub wetland ecological community have been lost (Figure 25), along with 41% of the cattail marsh ecological community (see Table 8, p. 40).381
Figure 25: Loss of water birch /roses riparian shrub wetland in the Okanagan Valley since 1800.
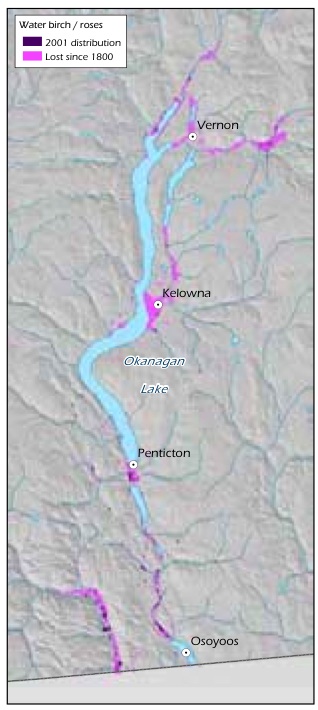
Source: Prepared for this report with data from T. Lea.
Data gaps: Found in narrow strips along watercourses and water bodies, riparian areas are dynamic ecosystems influenced by topography, surficial materials and stream flow, which are challenging to map and monitor, particularly at a broad scale. Data gaps include knowledge about disturbance and recovery regimes and the cumulative effects of disturbance regimes over space and time.382 Research on sediment production, terrain stability and channel stability is being undertaken in relation to watershed sensitivity in riparian areas.383 There are also gaps in the current knowledge about riparian soils, the role of trees in maintaining system integrity and system-scale properties of resilience.384
2.5.1.2 Terrestrial Elements
A. Decomposition And Nutrient Cycling
What is it? Decomposition is the process of breaking down the tissue of once-living organisms into their component parts. The breakdown of these building blocks is an important part of nutrient cycling. Brown rot fungi, which comprise one group within the suite of wood-decomposing fungi, were present 300 million years ago, when ancestors of conifers began to appear.385 They likely evolved along with conifers and influenced the evolution of coniferous forests, including the defence systems of trees that wall off the fungus, creating potential habitat for cavity-using vertebrates (see Section 2.5.1.2-F, p. 108). A more complex interaction involves fungi creating suitable substrates for bryophytes and lichens, which encourages the presence of various invertebrates and microbes, all leading to the gradual decomposition of wood.
Why is it important? Without fungi breaking down dead plant and animal matter, carbon and other molecules essential to life would be locked into organic molecules too large for plants to absorb. Fungi break down large organic molecules into inorganic constituents, such as carbon and nitrogen, which are small enough for the fungi to absorb; they do this by sending parts of their body (hyphae) directly into their food, secreting chemicals that help to break it down into simpler molecules, then absorbing the food directly into their cells. There is very little that fungi cannot or will not digest. Bacteria and arthropods help fungi break down organic compounds, but fungi are the engine. Organic compounds can be very complex, so it takes a suite of fungi and their enzyme systems to decompose wood, and other suites to decompose fur, feather, insects and dung. An example of nutrient cycling in the freshwater realm is described in Section 2.5.1.3-F (p. 121).
Status/threats in B.C. Decomposition proceeds almost unnoticed in the natural world at all times. However, this process is so fundamental that we will almost certainly notice if a significant change occurs. Climate change is causing temperature increases and changes in moisture availability, which will affect the rate of decomposition.388 This in turn will affect the raw materials necessary for nutrient cycling. Acidification is another factor affecting organisms involved in decomposition.389
Data gaps: There is little information on decomposition and nutrient cycling in B.C. ecosystems. Many organisms and pathways involved in decomposition and nutrient cycling are poorly understood.
Text Box 15. Mycorrhizae: A Tree's Best Friend
Fungi on plant roots form complex, mutually beneficial associations called mycorrhizae. They aid the roots of almost all vascular plants in several ways. For example, they increase the surface area of the roots dramatically. One centimetre of root has about 3 m of hyphae, effectively increasing the root's length by 300 times. This increases the surface area for absorption of water. One cubic centimetre of soil may contain 1 km of mycorrhizal fungal hyphae,386 with a fungal surface area of 300 cm2 interfacing with the soil. Through this increased surface area, the fungus actively and selectively absorbs minerals that the plant needs and transfers them to the plant, while excluding minerals that the plant does not need. Mycorrhizal fungi also secrete growth factors that stimulate root growth and branching, as well as antibiotics that protect the root from pathogenic bacteria and fungi. Nutrients and water in the soil are limited, and each root is surrounded by competitive bacteria, fungi and animals (nematodes), making mycorrhizal fungi extremely important to the health, growth and function of roots. In return for their services, they receive carbohydrates from the host plant.
Mycorrhizal fungi can be ectomycorrhizal, forming a sheath around the root tip of the host plant and growing on the outer surface of the roots, or endomycorrhizal, growing inside the roots. Most fungi on conifers are ectomycorrhizal; western redcedar and bigleaf maple (Acer macrophyllum) have endomycorrhizal fungi. Recent research has shown that in clearcut openings, ectomycorrhizal abundance and diversity decreases with distance from a stand edge and is higher next to old-growth stands.387
B. Pollination
What is it? Pollination is the transfer of pollen between plants by biological organisms or by abiotic factors such as wind.390
Why is it important? Flowering and seed-producing plants rely on pollination to reproduce. In natural and semi-natural habitats, 65-90% of plants rely on animals for pollination; the others rely on wind, water or splashing raindrops. Pollinating animals are rewarded, usually with food, often nectar. Vertebrates (mainly birds and bats) do some pollinating, but the majority of pollinators are insects: beetles, bees, wasps, flies, butterflies and moths. Insects pollinate more than three-quarters of all staple food crops; one of every three bites of food we take is a result of successful plant pollination by animals.391 While many larger trees (e.g., conifers, aspens and cottonwoods [Populus spp.], birches [Betula spp.] and alders are wind-pollinated, most plant species rely on the more precise delivery of pollen by animals. Close to the forest floor, wind is less prominent and animals are a more reliable means of pollination. The loss of pollinators, and in turn, the plant species they pollinate, would have cascading ecological effects.392,393 Insect pollinators are involved in food production for wildlife, and their larval stage is often an important prey item.394
Status/threats in B.C. We do not know the extent to which pollinators are of conservation concern in B.C. A study of the effects of landscape changes on bees in Garry oak ecosystems is currently underway.395,396 In other parts of the world (e.g., Hawaii, eastern Canada, southern U.S.) there are examples of individual pollinators and their host plants being severely affected. In the United States, major declines in both managed and wild populations of pollinators have been reported.397 A steady decline in insects has been associated with the overall decline in biodiversity as a result of ecosystem degradation.398 European honeybees (Apis mellifera), which typically have been introduced and managed as crop pollinators, are declining in abundance due to a variety of diseases and the impact of other alien species (e.g., the honey bee mite [Varroa jacobsoni]).399 Native bee communities can provide full pollination services where farms are located close to high value habitat. However, in areas where insecticides, herbicides and inorganic fertilizers are employed, where natural borders around agricultural fields are ploughed or where flood irrigation is used, agricultural intensification can reduce the diversity and abundance of native bees.400 These practices reduce floral diversity and habitat. Climate change, disease and parasites have also had negative impacts on pollinators.401 Ecosystems that are dependent on insect pollinators, such as alpine or subalpine meadows, are in more danger of decline than those that are primarily wind-pollinated such as grasslands.
Data gaps: We know little about native pollinators in B.C., whether or not their abundance and diversity are declining, and whether they are being displaced by the less-effective European honeybee, as they have been elsewhere.
C. Large Mammal Predator-Prey Dynamics
What are they? Predator-prey systems involve interactions between predators and their prey and occur at all scales, from interactions between invertebrates such as mites to those involving large mammals. Large predators do much to sustain the integrity, richness and productivity of terrestrial ecosystems.402,403 Unlike the predator-prey systems of small organisms, those of large species are reasonably well documented. B.C. is exceptional among northern temperate regions in retaining these intacta or relatively intactb systems, which have been lost in many other jurisdictions owing to significant population declines of large carnivores and ungulates (see also Section 2.5.2.2-B, p. 144). One important example of a predator-prey system in B.C. involves the grey wolf (Canis lupus) and ungulates such as deer, moose (Alces americanus) and elk.
Why are they important? Top carnivores often shape the structure and function of ecosystems by influencing the number, distribution and behaviour of their prey, including large herbivores, as well as the number, distribution and behaviour of smaller, generalist predators.404 Large herbivores shape the structure and species composition of plant communities, mainly as a result of foraging.405 Ungulate herbivory regulates the populations of their food plants and in turn, the stand-level structure of ecosystems. The grey wolf is one well-studied example of a top predator that has the potential to regulate prey communities of ungulates.406,407 Because wolves have the ability to increase rapidly, their populations can have significant impacts on the rest of the ecological community.
Changes in predator populations can shift prey population densities, prey community diversity and the distribution and abundance of ungulate forage vegetation. For example, the extirpation of wolves from Yellowstone National Park in the U.S. around 1925 (together with restrictions on the hunting of ungulates) is thought to have resulted in landscape simplification, such as the loss of riparian habitat caused by over-browsing by deer, elk and moose, and the loss of trembling aspen from the forest canopy. The reintroduction of wolves to the Yellowstone ecosystem has caused a cascade of effects, including the recovery of aspen due to a reduction in browsing by elk. 408,409 In the Yellowstone ecosystem, high moose densities resulting from the extirpation of both wolves and grizzly bears decreased bird species richness and nesting density through the reduction in riparian plants such as willows.410, 411
a An intact predator-prey system is one in which all of the native species are present, and with no alien species that plays a role as either predator or prey relative to the others.
b A relatively intact predator-prey system is one that is missing only one species, and with no alien species that plays a role as either predator or prey relative to the others, and where the loss of the species has not substantially altered the importance of predator-prey interactions to the populations of the remaining species.
In areas of B.C. where large carnivore densities are reduced by either disturbance or direct human-caused mortality, ungulate herbivory often exceeds sustainable levels and has deleterious effects on ecosystems and on other species. In the Rocky Mountain Trench, intensive ungulate herbivory in the relative absence of predators has contributed to overgrazing, which in turn has created opportunities for the spread of invasive alien species.412 Black-tailed deer, introduced to Haida Gwaii/Queen Charlotte Islands, where wolves are not found, have had major impacts on other species and resulted in the simplification of the forest ecosystem.413,414 Similarly, fallow deer (Dama dama) on Sidney Island near Vancouver Island have significantly affected the structure of the forest, creating a visible 'browse line' below which there is little evidence of palatable plant species. An overabundance of ungulates can also affect nutrient cycling, net primary production and fire regimes.415,416,417
Status/threats in B.C. Predator-prey systems cover most of B.C. and have only been lost entirely from areas of ecosystem conversion (see Section 3, p. 155). However, these systems have been directly impacted by: disturbance and fragmentation associated with motorized access,418 including off-road vehicles; killing of large carnivores as a result of, or with the intention of preventing, livestock-related conflicts with humans; predator control intended to increase ungulate populations; and/or hunting and trapping of large carnivores and ungulates. Predator-prey systems have also been indirectly impacted by the increase in young forests resulting from forest harvesting, which has increased moose and deer densities in some areas and, by extension, wolf densities.419
Data gaps: Interactions between some large mammal predators and prey species have been relatively well studied, but the dynamics of predator-prey systems are not as well understood. We know little about the indirect ways that human activities may influence these systems by creating advantages and disadvantages for individual species. For example, we do not fully understand the complex relationships between mountain caribou - a species of conservation concern - and other large mammals, but there is some evidence to suggest that landscape-level changes in forest structure (see Section 3.3.3, p. 194) are resulting in higher moose populations, which lead in turn to higher wolf populations and higher predation rates on mountain caribou (see Text box 13, p. 85).420,421,422
D. Succession/Disturbance
What is it? Succession is a series of dynamic changes in ecosystem structure, function, and species composition over time, as a result of which one group of organisms succeeds another through stages leading to a potential natural community or climax stage.423 This natural process occurs constantly in the environment. Organisms have an optimal environmental range for growth and development. As they become established on a site, they produce changes in soil conditions, micro-climatic conditions and physical space. 424 As conditions change, other species whose requirements are more suited for the new conditions will flourish. Each successional stage is dominated by a different combination of organisms. While the seral stages of vegetation can be broken into convenient units, the process is actually a continuum. A series of disturbances will result in an ecosystem that is generally composed of numerous patches of various sizes at different stages of successional development.425
A disturbance, whether natural or human-generated, can reset the successional process. Disturbance mechanisms such as fire, insect infestation, wind storms, landslides, flooding and logging are agents or enablers of succession and can interact together to influence succession. For instance, fire may make vegetation more susceptible to disease, while infestations of insects such as mountain pine beetle may increase fuel loads, making the area more susceptible to higher-intensity fires, resulting in significant changes to the existing successional regime.
Disturbance affects communities at various spatial and temporal scales. Disturbance agents and the frequency of disturbance can be recognized in the history of some ecosystems. Frequent fires in B.C.'s interior have resulted in open grassland savannahs with scattered large, fire-resistant trees. Lack of fire in moist-climate ecosystems has created a very different forest, with thousand-year-old trees growing next to young saplings. In these forests, the primary disturbance event is an old tree falling and creating a patch of sun. On floodplains, frequent flooding creates a constantly shifting mosaic of sediment islands and young trees and shrubs. Some species survive the disturbance and others quickly colonize the disturbed area.
Fire
Fire is a key disturbance agent, controlling forest and grassland ecosystem composition, structure and function.426,427,428,429 The way fire acts in an environment, referred to as the fire regime, has a major effect on the successional patterns of ecosystems.430,431,432,433 Where fire is suppressed, even in semi-desert ecosystems in British Columbia, conifers can invade grasslands.434
Fire regime is characterized by fire size, type, intensity and frequency, with each factor playing a role in ecosystem response to the disturbance event.435, 436 Figure 26 shows the variability of fire-return intervals in British Columbia. Four general natural disturbance types (NDTs) have been identified in relation to intensity and frequency of fires: rare or infrequent stand-initiating or crown fires (NDTs 1 and 2); frequent stand-initiating fires (NDT 3); and frequent surface fires (NDT 4). The fifth category on the map (NDT 5) shows non-forested areas that do not rely on fire to maintain that condition. At a more site-specific level, studies have shown considerable variability within these broad zones.437 Some wet coastal forests have been completely replaced through small gap-creating events during their thousands of years of existence in the absence of fire.438
The characteristics of a fire depend on many factors, including vegetation, fuels, weather and topography. For instance, grasses and conifer needles on the forest floor are important vectors for fire spread, while larger fuels and duff contribute to longer, more intense fire. Areas of high precipitation are less likely than arid areas to burn, and wind can direct the spread and duration of fire events. Steep, south-facing slopes and ridgelines are more prone to fire than shallow slopes or valley bottoms.439,440
Tree stand age also plays a role in fire regime, since a stand's fuel load, including the number of dead and down trees, generally increases with stand age. There are also important seasonal effects on fire type and vegetative response to fire.441
Fire regimes can determine the age structure of tree species within stands, which will, in turn, determine the types of plants and animals a community can support.442 Although some ecosystems, such as alpine ecosystems, have evolved largely in the absence of fire, others require fire to survive in their present form. Grassland and dry forest types have historically experienced frequent stand-maintaining fires.443
Figure 26: Distribution of natural disturbance types in B.C.
Source: B.C. Ministry of Forests and Range.
Wetter ecosystems experience infrequent, stand-replacing fires.444 Some individual organisms also depend on fire to fulfill a requirement within their life cycle.445 Depending on severity, fire can increase soil nutrient availability or sterilize the soil and destroy the successional seed stock.446
Insects
Insects can have a significant impact on succession. Various species of bark beetlesa collectively destroy more standing timber in western coniferous forests than all other insects combined. Pines, spruces, firs (Abies spp.) and hemlocks (Tsuga spp.) suffer most of the attack, in the order named.447
Adult bark beetles bore through the bark of conifers, making a tunnel between the bark and wood, in which to lay eggs. As the cambium-mining larvae grow, they create tunnels on the inner surface of the bark. Bark beetles are present in virtually all mature forests. However, under conditions favourable to the insects, major outbreaks can develop. Outbreaks that continue for many years can destroy trees over extensive areas. The most aggressive bark beetles are the pine beetles (Dendroctonus spp.)448 (See Text box 16, p. 105).
The longhorned beetlesb include some bark-boring species that can kill live trees or that may breed in the bark of felled, fire-killed or wind-thrown trees.
Why is it important? Disturbances such as fire and insect attack have a major effect on successional patterns and the structure and composition of ecosystems. When patterns of disturbance change because of either direct human activities or climate change, the resultant ecosystems may not function as expected.
Status/threats in B.C. Forestry practices, including fire suppression and salvage logging, have altered successional patterns.449,450,451 As a result of all these changes, forests are becoming less diverse, and therefore less resilient, and grasslands are declining.452,453,454,455 Climate change will further modify disturbance regimes (e.g., by increasing insect outbreaks) and cause large, sudden changes in vegetation.456
a Family Curculionidae, subfamily Scolytinae.
b Family Cerambycidae.
Text Box 16. The Mountain Pine Beetle Epidemic In B.C.
The mountain pine beetle is a natural part of B.C.'s interior pine forest ecosystems, laying its eggs under the bark of mature pine trees. When the eggs hatch, the pine beetle larvae feed on the inner bark, cutting off the tree's nutrient supply.457 The beetle also introduces a blue stain fungus into the sapwood, which prevents the tree from repelling the attacking beetles with pitch flow. A mountain pine beetle infestation can kill a host tree within a few weeks.458
B.C. is currently experiencing the largest mountain pine beetle infestation ever recorded in the province. The outbreak is due to two factors: the large amount of mature pine - the beetle's preferred host - present in B.C.'s interior forests as a result of past fire suppression and silvicultural practices; and the lack of sufficiently long periods of winter temperatures cold enough to kill overwintering beetles and keep the population in check.459,460 Drought during the past several years has also made the trees less able to resist attack.461
As of 2006, 19% of B.C.'s forest had been affected by mountain pine beetle, with an additional 32% expected to be affected by 2018 (Figure 27). The resulting changes in forest structure have a number of ramifications. Pine obligates, such as the western pine elfin (Callophrys eryphon) and the pine subspecies of the red crossbill (Loxia curvirostra stricklandi) may suffer from the dramatic loss of pine in the province.462 Loss of mature forest canopy reduces habitat quality and quantity for many bird and mammal species, and winter cover for ungulates, although the increase in standing and fallen dead trees will initially provide habitat for woodpeckers and cavity-nesting birds,463 and new understory vegetation may benefit some species, such as grouse, deer and bears. Reduction in transpiration as a result of extensive tree death is expected to significantly change forest hydrology.464 The resulting increased runoff will have short-term effects on stream systems, including erosion. The effects of increased logging levels aimed at salvaging beetle-killed trees may also be significant.465,466,467,468 The risk of fire in beetle-killed forests is increasing. The effects of climate change have already expanded the amount of habitat suitable for mountain pine beetle by 75% in the past three decades, and the beetle has now moved into Alberta. This trend is expected to continue (Map 10).469
Figure 27: Forests in B.C. affected by mountain pine beetle (MPB), with projections to 2018.
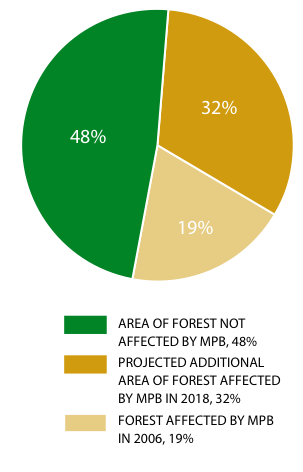
Source: Forest area is based on Baseline Thematic Mapping (BTM), Integrated Land Management Branch, from mid 1990s inventory data. Mountain pine beetle data are based on the Ministry of Forests and Range 1999 to 2006 Provincial Aerial Overview of Forest Health (www.for.gov.bc.ca/hfp/health/overview/overview.htm) and output from the BCMPB Projection Model (version 4) (www.for.gov.bc.ca/hre/BCMPB).
Data gaps: Regional disturbance regimes have been characterized for only 45% of the 91 biogeoclimatic subzones in the province.470 Most research has been focused on fire, with limited work on insects, wind or other disturbance agents. The lack of quantitative data is most likely related to the province's great diversity of ecosystems, which creates a much more complex system than is characterized by the four forested natural disturbance types described for B.C. This system is based on disturbance intervals and does not consider disturbance severity.471
Because insects that cause infestations in forests have large economic impacts they have been studied more than other insects. However, with increasing temperatures, particularly in the winter, the range of many species is changing. Monitoring these range changes will be important for predicting large-scale disturbances in the future.
E. Southern Red-Backed Voles
What are they? The southern red-backed vole is an herbivorous rodent distributed throughout much of B.C.
Why are they important? Southern red-backed voles play a number of functional roles in older forest communities, with influences on both their predators and their prey.472,473 They are prey for many species, including medium-sized mammals, forest hawks and owls. They facilitate nutrient cycling, including the decomposition of coarse woody debris, by dispersing a significant volume and diversity of fungal species (in one study more than 23 genera of fungi),474 many of which are mycorrhizal (see Text box 15, p.98). They aerate the soil by digging tunnels and they can influence local tree mortality by foraging on the roots of saplings and small trees, resulting in an increased mix of tree species in a stand and thus enhancing the number of niches available for other species. This can potentially contribute to the overall 'old-growth' quality of a stand.475 This species is known to be associated with stands that have higher densities of large pieces of coarse woody debris and of 'truffles' (edible underground fungi).476
Status/threats in B.C. Although the southern red-backed vole is not a species of conservation concern in the province, the subspecies occidentalis is critically imperilled and the subspecies galei is vulnerable. Southern red-backed voles are primarily associated with old-growth and mature forests,477 which are being reduced in distribution in many areas of B.C. Populations of these voles decline significantly with clearcutting.478 This species may be affected as old-growth forest is replaced by young seral forest and, over the long term, as the size and abundance of coarse woody debris declines across the landscape.479
Data gaps: We do not know to what extent this species' functions are replicated by other small mammals in younger seral stages, or how southern red-backed vole population cycles affect the dependence of any particular community on this species' functions. The loss of coarse woody debris across the landscape will intensify as more older stands become part of managed forests with short harvesting rotations. The ability of forest stands to maintain populations of the southern red-backed vole and associated species under those conditions is not known. No provincial population status information exists for this species.
F. Wildlife Trees
What are they? Wildlife trees are standing dead or living trees with special characteristics that provide nesting, denning, feeding, roosting or perching sites for wildlife species. The characteristics of wildlife trees include large trunks (sometimes hollow), large branches, deformed or broken tops, internal decay and sloughing bark.480 The interactions between fungi attempting to decompose living trees and the trees' defences produce different decay patterns, as the tree attempts to seal off the pathogenic fungi. Wildlife trees usually develop over a long time and the most important are usually large, old, damaged, diseased or decaying trees.
Why are they important? Many vertebrates profit from the pockets of rot inside a living tree resulting from the work of fungi. Wildlife trees provide habitat for at least 90 species in B.C.481 Some bird and mammal species rely on wildlife trees. Large platforms created by branches are used as nesting sites for large birds. Dead tops are used as hunting perches and nest sites. Trees with softened trunks are used by primary cavity nesters (those that build their own nesting cavity) and the abandoned holes are used by many other mammals and birds (secondary cavity nesters). This close community relationship between wildlife trees and primary and secondary cavity nesters has been described as a nest web.482 Some birds nest and bats roost under loose bark. Insects attracted to these trees feed a variety of birds. Black bears den in hollow trunks. Wildlife trees are especially valuable in grasslands, riparian areas and wetlands and along shorelines.
Status/threats in B.C. Wildlife trees are becoming increasingly scarce as old forests are harvested for forest products or cleared for agriculture and other types of land development. Forest harvesting rotation periods of 40 to 120 years do not allow enough time for wildlife trees or their diverse invertebrate communities to develop.483 Wildlife trees are sometimes classified as 'danger' trees and cut down for worker safety and are also often taken during salvage or firewood logging.484
Data gaps: Relatively little is known about the biology and ecology of many wildlife tree-dependent species, including the American three-toed woodpecker (Picoides dorsalis), black-backed woodpecker (P. arcticus), whiteheaded woodpecker (P. albolarvatus), Lewis's woodpecker, Williamson's sapsucker (Sphyrapicus thyroideus), flammulated owl (Otus flammeolus), western screech owl (Megascops kennicottii), northern hawk owl (Surnia ulula), northern pygmy-owl (Glaucidium gnoma) and northern saw-whet owl (Aegolius acadicus). Associations between wildlife trees and both lichens and invertebrates are very poorly documented. These gaps make it difficult to determine the effects of the loss of wildlife trees from ecosystems and to monitor the effectiveness of wildlife tree retention practices.
G. Broadleaf Trees
What are they? A broadleaf tree is one with relatively wide, flat leaves (as opposed to the needle-like leaves of conifers); in B.C., most broadleaf trees are deciduous, shedding their leaves in autumn. There are at least 17 species of broadleaf trees in B.C. and many more shrubs with similar characteristics. Some of the more common ones are red alder, trembling aspen, paper birch (Betula papyrifera), bigleaf maple and the province's only cottonwood - a subspecies of balsam poplar known as black cottonwood. Broadleaf trees are distributed in groups or singly throughout the forested zones of B.C. and, depending on the species, may be associated with riparian ecosystems.
Why are they important? Despite covering less area in B.C. than conifers, broadleaf trees are used for breeding habitat by more species, including a wider range of birds and mammals. They also provide important foraging habitat for many birds and mammals, and therefore presumably for a wide diversity of invertebrate species. Trembling aspen and paper birch, as well as other trees, shrubs and herbs, are associated with soil microbes and ectomycorrhizae, which colonize the roots of plants and form a network of fungal hyphae linking the broadleaf trees with neighbouring conifers in mixed forests (see Text box 15, p. 98). When deciduous species are present, carbon transfer and nitrogen fixation in conifers increases, suggesting that the broadleaf species are an essential component for maintaining longer-term ecosystem productivity.485,486 Broadleaf trees prevent the spread of the root disease Armillaria ostoyae among conifers487 and reduce attack by weevils and spruce budworm. These species also directly increase productivity of soil by dropping large volumes of leaf and branch litter onto the forest floor every year. Among the notable broadleaf tree communities in B.C. are black cottonwood ecosystems. Cottonwoods are important wildlife trees (see Section 2.5.1.2-F, p. 108) because they are prone to limb breakage, creating large natural cavities, and because many woodpeckers nest in them and are later followed by secondary cavity nesters. Cottonwood ecosystems are often, though not always, located adjacent to water bodies. They provide habitat for a wide diversity of animals, including species requiring large nesting trees close to water (e.g., great blue heron [Ardea herodias], bald eagle [Haliaeetus leucocephalus]), as well as black bears and many cavity-nesting ducks and owls. The large size and relatively short lifespan of cottonwoods means that they likely contribute significantly to riparian functioning, with leaf fall providing important organic matter, and inputs of large instream woody debris increasing habitat for fish spawning (and other aquatic breeding species) through pool development.
Ecologically, floodplain cottonwood ecosystems tend to include a diversity of ecological communities that are uncommon in B.C., including some of conservation concern. These ecosystems contribute to the structural integrity of stream bank and lakeshore habitats. In many locations, cottonwood ecosystems exist within the flooding zone of streams, rivers and lakes. This species (along with trembling aspen) is thought to act as a nutrient pump in forested ecosystems, whereby nutrients transported by these species into the canopy are released and made available to support other species, such as certain lichens that are found in greater numbers in areas where nutrients are made available by this 'drip zone' effect.488
Status/threats in B.C. In some areas, certain forest management practices (e.g., extensive brushing and chemical removal of deciduous species, fire suppression across the broader landscape) have resulted in there being too few young broadleaf trees, both within and outside the managed forest landscape, to replace existing mature broadleaf trees that are close to their natural lifespan of 100 to 150 years.489 In the short term, the loss of a mature component of broadleaf species will result in significant reduction of habitat for a diversity of species, including woodpeckers and associated secondary cavity nesters. In the longer term, loss of these trees from regenerating forests, both managed and natural, may have significant productivity impacts due to reduced nitrogen fixation from the loss of mycorrhizal fungi.490
Across the province, many cottonwood ecosystems are of conservation concern.491, 492 The B.C. Conservation Data Centre lists 12 ecological communities of conservation concern that include black cottonwood.493 Historic losses of cottonwood ecosystems have been significant in some areas. For example, in the Okanagan, 63% of the black cottonwood /water birch riparian shrub forest has been lost since 1800 (Figure 28).494 Losses are due to a number of factors. Damming of rivers has flooded areas historically dominated by cottonwood ecosystems.
Single trees and whole ecosystems are also affected by rural or recreational development and agriculture. In addition, regeneration of the species, and therefore the ecosystem, has been reduced in many areas because of reduced water levels and reduced flooding potential around many lakes (e.g., around the West Arm of Kootenay Lake),495 since black cottonwoods primarily regenerate after flooding. Climate change also has the potential to influence the distribution of the species through the expected significant increase in frequency of low water flows and reduction in peak flows.
Data gaps: Inventory of broadleaf trees, which have typically been considered non-commercial, is limited. The last inventory in the province was undertaken in the early 1990s, although a new inventory is underway. Black cottonwood is mapped on forest cover maps, but the distribution of the broader riparian ecosystem is not systematically monitored in B.C. No systematic mapping is available for cottonwood ecosystems located on private land, which may be significant. Current forest inventory sometimes omits small occurrences of broadleaf trees.
Figure 28: Loss of black cottonwood /water birch riparian shrub ecosystem in the Okanagan since 1800.
Source: Prepared for this report with data from T. Lea.
H. Soil
What is it? Soil is the naturally occurring, unconsolidated mineral or organic material at the surface of the earth that is capable of supporting plant growth.496 Different soil types across B.C. are the result of the five factors of soil formation: parent material, climate, organisms, topography and time. Variations in soil properties, such as texture, thickness and mineralogy, are inherited from the parent material; other properties, such as organic content, depth of the weathering zone, and the development of horizons (layers), are the result of soil-forming processes. The four major soil-forming processes - additions, losses, translocations and transformations497 - are primarily influenced by temperature, precipitation and organisms. Soils are classified by their properties, many of which reflect how the soils are formed. The type and variety of soils found across the landscape both influence, and are influenced by, biodiversity.
Why is it important? Soil represents the dynamic interface between the living organisms, air, water and rock. It supports plant growth and is fundamental to terrestrial ecosystems, including wetlands. Soil consists of mineral material originating from the parent material and organic matter formed by the decomposition of organisms and their by-products. Organic matter provides nutrients to plants, enhances the ability of the soil to hold water and enhances soil structure. It also provides aeration and drainage, promotes long-term site productivity and stores carbon.498 Soil is created by, and provides habitat for, highly complex bacterial and fungal communities, which are instrumental in decomposition and nutrient cycling (see Text box 15, p. 98), as well as diverse communities of invertebrates (e.g., nematodes, roundworms and arthropods), which act as detritivores and provide a food source for ground-foraging vertebrates such as shrews (Sorex spp.). Soil also influences larger animals, whose uses of soil include burrowing, nesting, travelling, cooling and obtaining minerals from mineral licks.
Soil is critical to future ecological response to climate change. Enduring landscape features such as parent material and topography will remain essentially the same as climate, biota and disturbance regimes change. Soils change over a longer time scale than individual plants and animals and retain characteristics and clues to past ecology and disturbance (e.g., floods, charcoal from forest fires, excavation, landslides, volcanic deposits and pollen). Different soil types may also act to buffer or amplify climatic, anthropogenic and ecological changes.
Status/threats in B.C. From a global perspective, soils in B.C. are generally believed to be in good condition, as impacts tend to be localized. The human activities that have had the largest impacts on soil are urban development, mining, forestry, grazing, creation of reservoirs and oil and gas development. Rural development and agriculture convert ecosystems, and the soil that remains tends to have much reduced value to biodiversity.
Forestry is one of the most widespread human activities affecting soil in B.C. The primary impacts from forestry are compaction through the construction and use of roads and landings, loss of soil material due to destabilization and hydrological processes, the resulting increased sedimentation in streams, and the reduction of organic matter inputs. Most organic material taken to a landing or mill is material that eventually would have become part of the soil. Grazing can also result in soil compaction and/or erosion, especially in riparian areas. Oil and gas development results in compaction through the construction and use of roads, well sites, pipelines and other facilities, as well as environmental contamination of soils in localized areas. Oil and gas drilling waste includes petroleum hydrocarbons.499
Also impacted by livestock trampling is a unique and rare type of soil cover known as the cryptogamic crust (also known as the microbial, microfloral, microphytic or cryobiotic crust) - a thin layer of lichens, mosses, liverworts, algae, fungi and bacteria that is found in undisturbed semi-arid ecosystems. It can be found on either soil or non-soil surfaces. The cryptogamic crust layer is important to water retention in the arid parts of the province.500 The cryptogamic crust in grassland communities in low-elevation areas of B.C. has mostly disappeared as a result of ecosystem conversion. In the isolated pockets where the cryptogamic crust still occurs and livestock have access, they can trample the crust and break it up.501
Data gaps: Because soil organisms are often microscopic or out of sight, there is widespread lack of information about them (See Text box 14, p.88). Some of the most obvious gaps are: knowledge of basic biodiversity found in soils, including inventories for single-celled organisms, fungi and microfauna; lack of taxonomic expertise to carry out these inventories; knowledge of specific functions of soil organisms, including how they are involved in maintaining specific physical and chemical conditions and how they contribute to ecosystem resilience and stability; knowledge of how climate change will affect the redistribution of soil organisms and the resulting impacts on soil processes; and knowledge of impacts of large-scale human disturbances (e.g., forest harvesting, fertilization, urban and rural development) on soil biodiversity and related functions.
Soils inventory mapping covers less than 50% of the province. For provincial-level modelling, 100% coverage would be desirable. Provincial soils data were collected between the 1930s and the early 1990s. Soils data are not easily accessible and are in a variety of formats.
Shallow soils on rocky terrain are associated with distinctive ecosystems and are widespread within B.C. These soils have not been well studied or described.
I. Coarse Woody Debris /Large Woody Debris
What is it? Coarse woody debris (CWD) is large pieces of wood, generally greater than 10 cm in diameter, on or near the forest floor, and includes sound or rotting logs, stumps and large branches that have fallen or been cut (standing dead trees are discussed in Section 2.5.1.2-F, p. 108).502 In aquatic environments, this material is called large woody debris (LWD) or large organic debris.
Why is it important? Coarse woody debris is important in both forest and aquatic ecosystems for several reasons.503,504 CWD contributes to stand-level diversity in old-growth and mature forests, providing habitat for feeding, reproduction and shelter for many organisms including invertebrates, small mammals and amphibians. It provides nutrients and habitat for various bacteria and fungi (see also Section 2.5.1.2-A, p. 97), as well as saprophytic plants,a lichens and mosses that are important for decomposition and nutrient and water cycling. It also acts as a refugium for displaced species where it persists during and after disturbances, can buffer microclimates for the establishment of seedlings, and it stores carbon.
In aquatic and riparian ecosystems, LWD shapes and stabilizes streambanks and prevents erosion.505 When it falls into streams, it disperses stream energy and enhances fish habitat by creating pools, gradual steps, gravel bars and riffles. Large woody debris releases nutrients and increases the retention of organic debris from upland sources, which is then decomposed by stream organisms.
Status/threats in B.C. In the aquatic environment, the absence of LWD has a negative effect on the stability of stream structure and on the species that use it as habitat. Removal of wood from large rivers can result in altered channel structure and have long-term effects.506 Long and large-diameter pieces of CWD take longer to decay than smaller pieces and therefore add structure over a longer time period. They are rarer in harvested sites compared to natural areas.507,508
Data gaps: There are still many unanswered questions about the role of CWD in forested ecosystems and the ability of these functions to be maintained in systems with diminished piece size. In freshwater systems, particularly large rivers, there is a lack of information on the life cycle of LWD and its effect on fish habitat.509
2.5.1.3 Freshwater Elements
A. Wetlands
What are they? Freshwater wetlands are areas where fresh water is at or near the surface for most of the year. They include bogs, fens, swamps and marshes. Temporary wetlands provide habitat for a number of extremely rare taxa.510
a Plants that grow on, and derive their nourishment from, dead or decaying organic matter.
Why are they important? Wetlands provide habitat for many species and fulfill a broad range of ecological functions. The largest wetland complexes in B.C., such as the Columbia Valley wetlands (see Section 2.5.2.3-A, p. 145), are internationally significant. The province's more numerous small wetlands make major contributions to biodiversity;511 they cumulatively create wetland complexes (which are important features in many landscapes) and may be interconnected.512 Temporary wetlands (seasonally flooded wetlands that have both a wet and a dry regime, which vary in timing, frequency and duration) are important for amphibians513 and invertebrates,514 as well as for zooplankton and plant species.515
Wetlands buffer environmental extremes by absorbing and filtering sediments, pollutants and excess nutrients, recharging groundwater, maintaining stream flows, controlling runoff, storing flood waters, reducing erosion and stabilizing shorelines. They also help regulate atmospheric gases and climate cycles. In brief, wetlands absorb water quickly and release it slowly, with its quality improved. The contribution of wetlands to biodiversity and to ecological functions maintaining both terrestrial and freshwater systems is disproportionate to their size. Wetlands are areas of high species richness. A large proportion of B.C.'s terrestrial vertebrate species rely on wetlands to meet some of their needs or use wetland habitat at some point in their life cycle, and more than 30% of the province's species of conservation concern are wetland-dependent.516
Status/threats in B.C. Wetlands are estimated to comprise about 7% of the province's area, but are decreasing rapidly. Because they tend to occur in low-lying areas suitable for agriculture and settlement, wetlands are among the most heavily impacted ecosystems worldwide. They are subject to many impacts that reduce their suitability as habitat, including removal of water, siltation, pollution, water diversion, ecosystem conversion to urban and agricultural uses, and general human disturbance.
The Okanagan River is almost completely channellized, contributing to the loss of about 85% of the original wetlands in the south Okanagan.517 Many of the wetlands in the lower Fraser Valley have also disappeared, with the diking and drainage of 85% of the wetlands in the Fraser River delta518 (see Text box 7, p. 43) and the complete eradication of the once-rich Sumas Lake wetlands. Between 1989 and 1999, approximately 20% of the wetlands in the Fraser Valley between Hope and Vancouver were impacted by urbanization or agriculture,519 including peatland drainage in the Fraser River delta. On the east coast of Vancouver Island, there was a loss of approximately 32% of salt and estuarine marshes between the early 1900s and 1988,520 and a loss of 2% of freshwater wetlands between early 1990s and 2002.521 Agricultural reclamation of wetlands in the Cariboo-Chilcotin has contributed to ecosystem conversion and degradation in that region.522
Climate change could jeopardize shallow wetlands and their contributions in the B.C. interior, and rising sea levels could inundate coastal wetlands.523,524
Data gaps: Although the wetland and riparian ecosystem classification of British Columbia is well developed,525 not all of the province has been surveyed. Smaller wetlands are not well surveyed or inventoried. Temporary wetlands are not included in any surveys.
B. Sphagnum
What is it? Sphagnum mosses (commonly known as peat mosses) are bryophytes that inhabit and distinguish wetland ecosystems characterized by their acidic environment and an accumulation of organic matter (peat). In B.C., significant Sphagnum ecosystems are found in the boreal forests of the northeast and along the coast.526 Burns Bog is a well known Sphagnum-dominated bog found in southwestern British Columbia (see Section 2.5.2.3-A, p. 145).527,528
Why is it important? The morphological, chemical and physical properties of Sphagnum species contribute to the development of the wet, nutrient-poor, acidic environments in which they flourish.529 By raising the peat surface, acidifying its environment and absorbing 10 to 20 times its dry weight in moisture, Sphagnum has a disproportionate impact on its surrounding environment and plays a significant role in wetland succession.530 Sphagnum is required to fix the acidity of the bog ecosystem to allow the succession of other wetland species.531
Acidic and nutrient-poor environments created by peat mosses are unique ecosystems in which only a few specialized species are able to thrive. These ecosystems contain rare species such as the subarctic darner (Aeshna subarctica) and Pacific water shrew.532,533 Environments created by Sphagnum also provide habitat for waterfowl.
Peat bogs created by Sphagnum are of international significance for their role in mitigating the effects of climate change by trapping greenhouse gases such as CO 2 and methane, which otherwise would be released during decomposition processes.534 Sphagnum environments control run-off, act as catchment areas and provide repositories for natural and anthropological history preserved in organic layers.
Status/threats in B.C. Ecosystem conversion, predominantly through peat mining, is the main threat to coastal Sphagnum. Sites are generally small and therefore susceptible to disturbance. Clearing the top layers, such as for agriculture, releases methane, contributing to climate change. Flooding destroys the habitat. Adjacent agricultural development also has the potential to introduce chemicals, altering the environment and species succession.
Drainage allows other species to become established, and once bogs have been drained, natural regeneration of Sphagnum is difficult. Harvest is a threat to Sphagnum located near urban environments. Although these plants can cope with seasonal and daily surface temperature fluctuations and periodic drought, Sphagnum species are susceptible to long-term changes due to climate change. Repeated burning is not favourable to their survival. Oil and gas development is a threat to Sphagnum ecosystems in northeastern B.C.535
Data Gaps: There are significant gaps in knowledge of life history strategies, including dispersal, of Sphagnum species.536 Although they are found in locations with regular fire occurrence, the influence of fire on these plants is poorly understood.537 There is no systematic inventory of Sphagnum.
C. Lake-Level Patterns
What are they? Many lakes are characterized by predictable temporal water-level patterns.538 The regular pattern of lake levels is affected by several aspects of climate (e.g., precipitation, temperature, humidity) and by physical setting (e.g., geology, basin shape, basin size). Natural lake-level patterns vary considerably among geographic regions of the province. Many natural lakes in B.C. have water licenses allowing drawdown and others have been modified by the addition of weirs to manage lake levels. These changes create water bodies that are part natural lake and part reservoir. This key element includes these modified lakes, but does not include the entirely artificial water bodies created by dams.
Why are they important? Emergent and submerged aquatic macrophytes (large aquatic plants) are the dominant structural component of shoreline habitats. They provide food and shelter for a wide variety of invertebrate, fish and wildlife species. Vegetation responds to strong gradients between the terrestrial and aquatic interface and under most natural conditions there is strong zonation of vegetation along this gradient from forest and shrub thicket to wet meadow to marsh to aquatic.539 The wet meadow and marsh zones are found solely within the area affected by fluctuating water levels.
Fluctuating water levels are considered the most important factor determining vegetation patterns on lakeshores, although other factors, such as wave exposure, soil or sediment type, and species interactions are also influential. Under most natural conditions, water level fluctuations occur due to seasonal changes in precipitation and surface/groundwater inflows, as well as discharge and evaporation. Climate will influence precipitation and temperature (evaporation), while the physical setting will influence surface and subsurface flows.
As a lake's water level drops, wetted areas are exposed and the zone where light levels are sufficient for plant growth (the photic zone) shifts deeper in the lake basin.540 The frequency, duration and magnitude of drops in the lake level determine the effect on vegetation and some animal species. For example, lake sockeye salmon need stable lake levels for successful reproduction.541,542
Status/threats in B.C. Humans affect lake levels through dams, water diversions and extractions, and land use changes. We do not know the extent of these impacts and they may increase in response to climate change.
Climate change is already having noticeable effects on streamflow patterns in some areas of B.C., which will likely affect lake levels.
Altered lake levels affect the quantity and quality of shoreline and riparian habitats, and the abundance and distribution of aquatic and riparian organisms.543 As water level fluctuations increase in magnitude, there is often a decrease in the extent of the shoreline zone as high-elevation areas are inundated insufficiently to support emergent species and too frequently to support upland vegetation. Low-elevation areas are unproductive because the photic zone does not extend to these areas for enough time to allow establishment of submerged macrophytes.
Lake drawdowns of 5-7 m can completely remove macrophytes from the shoreline zone.544,545,546,547 Relatively minor shifts in average annual water levels (plus or minus 10 cm) or in water level fluctuations can produce substantial changes in the vegetation community, and species changes are common even after a single drawdown.a,548,549 Stabilized water levels are also capable of significant effects on the diversity of macrophytes in the shoreline zone.550
It has also been suggested that drawdowns in lakes can cause algal blooms (visible aggregations of algae in or on the surface layer of a water body) when nutrients are released from exposed shoreline areas, causing a subsequent limited uptake of nutrients in shoreline habitats.551,552 Shoreline zone invertebrate populations can be severely reduced by drawdown and many studies have shown correlations between fish abundance and diversity and altered lake-level patterns.553,554,555
Data gaps: The effect of water level and drawdown on macrophyte distribution and abundance has been fairly well studied, though results have been variable due to differences among studies in lake geology and physical characteristics, species affected and differences in drawdown scenarios.556
D. Headwater Streams
What are they? Headwater streams are found at the top end of stream systems. They are often steep streams dominated by bedrock sills and influenced by landslides that deliver boulders and other sediment to the channel. Headwater streams can represent half of the total length of a river system.557
a The magnitude of fluctuations is one of the primary differences between reservoirs and natural lakes, and causes extirpation of shoreline vegetation from most hydropower reservoirs.
Why are they important? These small streams are critical for input of water and sediments, for nutrient dynamics and for the addition of most of the coarse particulate organic mattera in stream systems. Most (70-90%) of the coarse organic particulates are transported downstream,558 where they provide a food source for stream invertebrates that in turn feed larger organisms. Headwater streams are strongly linked to adjacent slopes. Landslides account for most of the sediment in headwater channels.559
While steep headwater streams tend to be fishless, they provide habitat for many aquatic invertebrates, and low-gradient headwater streams are among the most productive environments in a river system because of the retention of organic material. For solely aquatic species (e.g., fish), headwaters can be geographically isolated from one watershed to the next. This means that they are also genetically isolated, which accounts for the genetic variability within these species between headwater systems.560
Status/threats in B.C. Although headwater streams make up more than half the length of a stream system, they are often so small that they are not mapped or considered in resource management, which puts them at greater risk than other streams.561 This is particularly true if they are not fish-bearing. Much of the structure and many of the processes within streams, including headwater streams, are determined by relationships with adjacent ecosystems, particularly the riparian zone. The status of adjacent riparian systems affects:
- microclimate (light, temperature and humidity);
- nutrient input from hill slopes (riparian zones modify amount, form and timing of nutrient export from watersheds);
- contribution of large woody debris (see Section 2.5.1.2-I, p. 113);
- contribution of organic matter (much of the food base for stream ecosystems comes from adjacent terrestrial sources); and
- retention of inputs to LWD jams and smaller organic debris accumulations.
Data gaps: The importance of headwater streams to downstream ecosystems is not well known. There is no measure of the amount of headwater area that has been degraded by roads, timber harvesting, mineral exploration and mining in B.C.
a Coarse particulate organic matter is particles of organic matter (leaves, wood, etc.) that are >1mm in size.
E. Groundwater
What is it? Surface waters in the form of rivers, lakes and wetlands are the most readily apparent component of the hydrologic cycle, but in most areas of B.C. there is a strong interaction between surface flows and groundwater (see also Section 2.5.1.1-B, p. 94). This interaction is apparent when surface flows in perennial streams continue long after precipitation or snowmelt runoff events. Groundwater is recharged by infiltration from precipitation and surface flow and, depending on the depth of the water table and subsurface geology, groundwater may be subsequently released as surface flow.562,563 The release of groundwater to surface water provides most of the base flow for many streams through periods of no precipitation, or during winter when precipitation is locked up as snow or ice.
Why is it important? A minimum flow is important to organisms such as fish, not only as a constant medium in which to live, but also because of the ameliorating effects on extreme temperatures by groundwater inputs.564,565 In winter, groundwater releases are typically warmer than ambient surface water and may prevent freezing conditions in spawning and rearing areas.566,567 In summer, groundwater is often cooler than the ambient temperature, which can help keep stream temperatures within the thermal tolerance limits of cold-water fish species.568 Many fish congregate near groundwater sources at different times of year. For example, bull trout sometimes use groundwater upwelling sites for spawning, and incubation at these sites is considerably more successful than in adjacent non-upwelling sites.569 Similar results have been reported for spawning salmon in lakes, streams and reservoirs.570, 571,572,573 Migrating adult chinook salmon sometimes take advantage of cool groundwater upwelling sites at times when many nearby stream locations are beyond their physiological thermal limits.574,575
The hyporheic zone is thought to be a critical refuge for surface-dwelling invertebrates, and most insect families and other groups that live in surface waters have also been collected from the hyporheic zone.576,577 Nutrients released to surface waters from the hyporheic zone influence surface water quality and productivity, to the point of creating productivity hot spots in some instances.578,579
Changes to groundwater resources have varying impacts on biodiversity, depending on the physical and ecological setting. Some species in the diverse hyporheic community, such as bull trout, depend directly on groundwater resources.580 Other species and communities are less dependent, but large and measurable adverse consequences are nevertheless likely. For the many species of fish that use groundwater upwelling areas as spawning habitat or holding habitats during migrations, loss of these features could reduce incubation success and raise physiological stress levels; both could potentially decrease the abundance of adult and juvenile fish and lead to replacement by more thermally tolerant species. Lower water tables can have profound effects on riparian and floodplain vegetation, with cascading effects on local biodiversity and physical effects, such as lower streambank stability.
Status/threats in B.C. Overall, groundwater resources are of considerable concern in a number of locales in B.C., particularly in the southern portion of the province where agricultural, municipal and industrial demands for groundwater are highest. Approximately 25% of B.C. residents obtain drinking water from groundwater sources.581 Thirty-five aquifers were designated as 'heavily used' in 2001, up from 17 in 1996.582 Monitoring data indicate that groundwater levels are declining in areas where groundwater withdrawal and urban development are most intensive.583
Groundwater and surface water can be influenced directly by a number of factors, including climate, land use, water use and industrial activities. How each of these act within a particular watershed is rarely straightforward, but there are many examples linking human activities to changes in quality and quantity of groundwater and surface water, and interactions between the two water sources. These include the following:
-
When groundwater is removed for human use there can be direct, measurable influences on stream flow.584,585
-
Changes to water table levels can significantly influence riparian vegetation.586
-
Surface changes, such as river regulation, sediment inputs or eutrophication, have a direct impact on water table levels by altering infiltration and exfiltration rates.587
-
Release of contaminants into surface waters can infiltrate and alter groundwater quality.588
-
Groundwater recharge rates are closely linked to temperature and precipitation, which are expected to change due to climate change.589,590
-
Impervious surfaces, such as pavement, can have a large influence on runoff and groundwater recharge, with effects on minimum and peak stream flows.591,592
-
In agricultural settings, changes in land cover, drainage and irrigation can have severe impacts on stream flows.593,594,595
Data gaps: There are large uncertainties about where groundwater is, how it moves, locations and rates of groundwater-surface water exchange, aquifer recharge rates, and impacts of land use, water use and climate change. In B.C., work is underway on hydrologic modelling and measurement of some high-value aquifers, and stream and lake ecologists are starting to incorporate knowledge of groundwater dynamics into their studies.
F. Anadromous Salmonids And Nutrient Cycling
What is it? Fish that breed in fresh water and spend at least part of their adult life in a marine environment (i.e., anadromous fish) play a significant role in nutrient cycling bringing marine nutrients to terrestrial and freshwater ecosystems. Anadromous salmonids found in B.C. are chinook, chum, coho, pink and sockeye salmon, and steelhead. Some other species are partially anadromous and are not included in this discussion.
Anadromous populations are found throughout the Fraser River system and almost all coastal systems. Historically, anadromous salmon reached Columbia Lake in the headwaters of the Columbia River, but they have been extirpated from the upper Columbia River due to impassable dams in the U.S. Anadromous populations are absent from the Kootenay River system due to natural barriers. They are also absent from the Peace River drainage, which drains east into the Mackenzie River system.
Why is it important? The majority of freshwater systems in B.C. are oligotrophic (i.e., nutrient-poor). This means that the inherent productivity of these water bodies is low and the abundance and biomass of fish that can be supported is also low. Anadromous salmonids act as a nutrient pump, bringing nutrients from the ocean to nutrientpoor interior waters. They hatch in freshwater streams (and occasionally in lakeshore areas) and migrate to the ocean after a variable amount of time in freshwater. After growing and accumulating most of their body tissue in the ocean, they return to their natal streams and lakes to reproduce and then die. Anadromous salmon may return in large numbers, and this returning biomass brings marine-derived nutrients into freshwater ecosystems and supports many terrestrial and freshwater species. They are a limiting food resource for a wide variety of vertebrates and invertebrates, including both predators and scavengers.596
Many adults are caught and consumed en route to or on the spawning grounds, eggs are eaten by birds and fish, and the carcasses of spawned-out salmon provide a substantial input of nutrients to many freshwater and terrestrial systems (Figure 29).597,598 When the young hatch the following spring and summer, they are the prey base for many more species. Streamand lake-rearing juveniles are often the dominant component of the fish community.
There is some evidence of positive feedback between present and future salmon runs due to the profound nutrient inputs provided by returning adults.599,600 The nutrients from the adults increase macroalgae production in estuaries, boosting the productivity of crustaceans, which become prey for salmon smolts. Lower returns lead to lower nutrient levels in lakes and rivers, which reduces the carrying capacity of these water bodies and thus the ability to support fish. Lower abundance or loss of this element would lead to lower abundance of many terrestrial and freshwater species and lower overall productivity in many freshwater bodies. Conversely, there is evidence that smolts returning to the ocean can represent a potential loss of nutrients from the freshwater system to the marine.601,602
Figure 29: The relationship between salmon returns, bears, riparian forests and future salmon productivity.
Illustration: Soren Henrich
Status/threats in B.C. The approximate number of populations for each species is: chinook salmon (780), chum salmon (1,450), coho salmon (2,400), pink salmon (2,100), sockeye salmon (900) and steelhead (850).603
In the mid 1990s, 5,487 British Columbia and Yukon anadromous salmonid populations were assessed; 19.6% of these were of conservation concern (i.e., high conservation concern, moderate conservation concern or special concern) or extirpated.604 Although the rest of the assessed populations were not below a threshold for conservation concern, many are likely below historic abundance levels. Many of the populations of conservation concern are small. Most of the populations that were not assessed are also small and are therefore likely to be of conservation concern. Many of the ecosystems with smaller populations may be becoming progressively oligotrophic due to declining inputs of marine-derived nutrient inputs.605 In the late 1990s, the sockeye salmon runs in the Owikeno Lake system of the central coast collapsed, with returns reaching a 50-year low in 1999.606 That fall there was a dramatic increase in the number of conflicts between humans and grizzly bears at the nearby community of Rivers Inlet. Ten grizzly bears were destroyed and three more were translocated.607 Monitoring from 1998 to 2002 documented a decline in grizzly bear use of salmon streams in the area and indicated that switching to alternative food sources such as berries is not a viable option for most bears when salmon abundance is low. These results suggest that the population declined in response to the dramatic reduction in this critical food source.608
Because of their complex life histories, anadromous salmonids are vulnerable to changes in biophysical processes or disturbance regimes that occur throughout the watersheds they inhabit. During the freshwater part of their life cycle, they are affected by harvesting, land and water use, pollution, alien species and climate change. Impacts such as riparian ecosystem conversion or degradation, alteration of drainage patterns and pollution are additive and may produce a large incremental effect if combined with other activities.609 Sources of ecosystem degradation include construction of dams and stream crossings, water impoundment, land clearing (related to urban development, agriculture or logging), mining and gravel removal. Increased runoff increases nutrient levels, erosion, and sedimentation in streams. Removal of riparian vegetation raises water temperature.610
Climate change is expected to have severe impacts on freshwater fish distributions in B.C. through effects on precipitation,611 streamflow612 and water temperatures,613 and range changes in native and non-native species.614,615,616,617,618 Pacific salmon are highly vulnerable to the effects of changes in freshwater flows and temperature. Low water flows in the late summer can block access to spawning grounds, while winter flooding can wash eggs out of the gravel. Rising water temperatures in the Fraser River are expected to delay sockeye salmon migration and reduce enroute survival. Thirty-two areas, centered in southwestern B.C. and the southern and central interior, have been identified as being uniquely vulnerable because they have water temperatures or low/high freshwater flows that currently affect salmon survival.619
Within the marine realm, B.C. salmon are affected by a number of impacts and threats, including harvesting, aquaculture and climate change. Harvest rates vary over time and among populations, but can exceed 50% for targeted populations.620 Harvest rates can be unsustainably high for populations of conservation concern that are harvested incidentally.621,622,623 Known and suspected impacts of marine aquaculture operations on wild salmon include competition from, and interbreeding with, escaped farm fish, and the spread of diseases or parasites such as salmon lice (Lepeophtheirus salmonis).624 Climate change is expected to influence several environmental variables that may affect salmon during the marine portion of their life cycle; of these, sea surface temperature is likely to be the most significant.625
Data gaps: The ecology of anadromous salmonids is well known and commercially harvested populations are well studied. However, there is limited information on smaller populations. The effects of land and fisheries resource management practices on salmonids and nutrient cycling are not well understood.626 There are also uncertainties regarding the nature and strength of interactions with other species and with ecosystems.
G. Willows
What are they? Willows are deciduous trees and shrubs in the genus Salix that grow in moist habitats from the high Arctic to the tropics.627 B.C.'s more than 48 native willow species range in height from tall trees to low, carpet-forming shrubs, and grow in habitats ranging from upland forest and alpine tundra to the full spectrum of wetland types.
Why are they important? Willows are common within riparian areas and swamps. They provide structure, reduce water flows, stabilize stream banks and are a food source for species such as American beavers (Castor canadensis), moose, snowshoe hares (Lepus americanus) and grouse (e.g., willow ptarmigan [Lagopus lagopus]).628 The specialized root system of willows allows them to thrive in conditions that are potentially limiting to other woody plants, such as the high water table in swamps. Willows grow prolifically in a variety of nutrient-poor ecosystems and have the ability to grow from broken branches.
Status/threats in B.C. Nine species and one subspecies are of conservation concern. All of the province's native willows are at the geographic margins of their ranges in B.C., with the core of their ranges to the north, east or south, so some species could be expected to be rare in the province. However, some may be more common than is recorded, since willows are difficult to identify and some occur in remote places. Glabrous dwarf willow (S. reticulata ssp. glabellicarpa) is only found in B.C. and Alaska, and is of global conservation concern.629 An introduced weevil, the poplar and willow borer (Cryptorhynchus lapathi), has spread widely in the past 30 years and has been killing willows at low elevations and latitudes.630 It is spreading north along highways and logging roads and is expected to extend its range north and to higher elevations with climate change. In B.C., this species primarily attacks young willow stems, which are often killed by larval feeding.631
Data gaps: The spatial distribution of willow species is not documented. The tendency of willows to hybridize creates complications in tracking species.
H. Beavers
What are they? The American beaver, North America's largest rodent, is a keystone species that plays a large number of functional roles in freshwater ecosystems and riparian forests. Because of their profound influence on the physical structure of the ecosystems they inhabit, beavers are sometimes called ecological engineers.
Why are they important? The beaver is the only non-human species that impounds water by constructing dams that flood or maintain flooding in significant areas. By cutting down large numbers of trees and shrubs, beavers change the course of succession and vastly influence ecosystems and species.632, 633 Impoundment by beavers can create wetlands and wet meadows, and, in the process, create habitat for other species. The subsequent flooding and killing of standing trees provides habitat for many cavity-nesting species and foragers. Over the long term, wetlands silt up to form highly productive meadows and riparian habitat. The process of damming reduces channel scouring and erosion, changes sediment loading and flow regimes downstream, recharges groundwater levels, regulates water flow and can create cold springs, resulting in better conditions for coldwater fish species such as trout. Beaver dams may also form warm-water pools.634
The removal or addition of beavers in a particular area will often profoundly change local habitat types. Removing beavers generally results in the loss of beaver dams in the short term (unless they have been established for significant periods and are maintained by stream processes) and subsequent loss of standing water, removal of habitat for associated freshwater and terrestrial species, and significant changes in downstream hydrology.635 This process will influence habitat availability for other animals, including terrestrial birds, mammals and amphibians and many aquatic species.636
Status/threats in B.C. In B.C., the estimated beaver population is 400,000 to 600,000 and this species is not of conservation concern.637 However, the habitats of the beaver - wetlands and free-flowing rivers - have been extensively impacted throughout lower elevations, particularly in the province's southern regions. Hydroelectric impoundment, rural and agricultural development, and forestry activities have affected historic habitat, and local populations have likely been impacted in these areas.638 Localized changes to beaver populations due to trapping have been observed, and habitat changes have resulted in local extirpation.639
Beavers are not native to Haida Gwaii/Queen Charlotte Islands and their introduction to these islands has caused significant habitat degradation, to the extent that water flow has been reversed across parts of Graham Island.
Data gaps: There are no significant data gaps. However, the potential impacts of small-scale hydro projects on beavers and their associated habitats are unknown.
I. Waterfowl Herbivory Of Aquatic Plants
What is it? In aquatic habitats, waterfowl forage on plants that are emergent (leaves above the water surface), submergent (below the surface) and floating.
Why is it important? Since waterfowl herbivory changes the competitive hierarchy of plant communities within a wetland, it affects species abundance and the composition of plant communities.640,641 A reciprocal relationship also exists, as the abundance and distribution of plants provides an important food source for waterfowl and, therefore, their abundance and species composition. In particular, waterfowl herbivory can be a regulating factor in submerged aquatic plant populations in shallow sheltered areas.642
Herbivory was only recently identified as an important factor affecting the community structure of macrophytes.643 Traditionally, the breakdown of aquatic plants into detritus was assumed to be the most important mechanism for bringing this plant material into the food web. It is now thought that waterfowl grazing plays major role.644
Status/threats in B.C. There is uncertainty about the relationship between waterfowl herbivory and the loss of freshwater ecosystems. The main threats are the loss of wetlands and the potential local overpopulation of some waterfowl species.
Data gaps: There is a lack of information about the magnitude of the effect of waterfowl herbivory on aquatic plants, and about spatial and temporal differences in these plants and waterfowl species in different parts of B.C.
2.5.1.4 Marine Overlap Elements
A. Macroalgae
What are they? Macroalgae are commonly known as seaweeds and include numerous species, such as those that constitute kelp beds or 'forests' (e.g., Nereocystis luetkeana, commonly known as bull kelp or bladder kelp). Kelp forests are usually found in a 100-m-wide band along the shore and are components of the intertidal zone in B.C.
Why are they important? Macroalgae provide habitat, particularly nursery areas for juvenile fish and invertebrates, and are the major primary producer in the shallow ocean margins.645 Organisms graze on them directly, they break apart and become detritus, and eventually become part of the ocean environment as dissolved components. They are also an important source of nutrients when they wash up on beaches.646 Kelp forests are large, three-dimensional structures that provide habitat and a significant source of food to marine ecosystems from the detritus of the abundant drift algae associated with kelp forests, thus supporting a large variety of invertebrates and fish.647,648,649,650,651 Kelp forests dampen wave heights and tidal currents, and can influence dispersal, settlement rates and recruitment of benthic invertebrates and rockfish.652
Status/threats in B.C. Macroalgae populations in B.C. are generally stable, but some changes in local distribution and abundance have been observed.653 The recovery of sea otter (Enhydra lutris) populations and climate change will influence macroalgae populations.654
Data gaps: Mapping of macroalgae on the B.C. coast is incomplete.
B. California Mussels
What are they? The California mussel (Mytilus californianus) is a large bivalve mollusc that forms extensive aggregations attached to bedrock on exposed intertidal shores (and is also subtidal at high-current sites). The California mussel is found from Alaska to Baja California.
Why are they important? This mussel, particularly when it forms extensive mussel beds, is critical to intertidal ecosystems as a primary consumer, prey for a number of species and provider of habitat for a large community of species. More than 300 other species inhabit the interstices of established California mussel beds.655
Status/threats in B.C. For the most part, California mussel populations in B.C. are stable and near historic levels. Across their range, mussels have declined in both number and size in association with proximity to human settlement, due to recreational harvesting. Declines of extensive beds of large mussels have been documented on the west coast of Vancouver Island, with a shift toward scattered patches of small individuals and an associated decline of predators and community abundance.656 As California mussels generally recruit to established beds, recovery from disturbance is slow.657 Ocean acidification is a threat to California mussels (Text box 17). Climate change may already be affecting populations in some areas outside B.C.658
Data gaps: Mapping of California mussels on the B.C. coast is incomplete.
C. Sea Otters
What are they? Sea otters are marine members of the weasel family that use the intertidal zone when it is inundated. Sea otters live in cold oceanic water, but have no body fat. They survive through a combination of a very high metabolic rate and highly insulative fur. Their metabolic rate is such that they consume between 23 and 33% of their body weight per day.659 Sea urchins (Strongylocentrotus spp.), where available, make up much of their diet, particularly where sea otters have been recently reintroduced and the sea urchin population is high.
Why are they important? Sea otters are a prime example of a keystone species.660 Where sea otters occur, sea urchin populations are small and confined to cracks in the substrate or under boulders.661 From these protected locations, sea urchins switch their feeding strategy from actively grazing kelp to passively feeding on the abundant drift algae associated with kelp forests.662 In the absence of sea otters, sea urchins suppress kelp forests with cascading ecological effects.
Status/threats in B.C. The sea otter is a species of conservation concern in B.C. Once abundant along much of the B.C. coast, sea otters were extirpated from B.C. by the early 1900s. Relocations from Alaska between 1968 and 1972 successfully established colonies in Checleset Bay (now protected as an ecological reserve) and Hakai Luxvbalis Conservancy Area.663 The sea otter has since repopulated 25-33% of its historic range in B.C., but is not yet considered secure, as populations are small (less than 3,500).664
Sea otters are threatened by oil spills, disease and parasites, low genetic variability, marine biotoxins, contaminants, entanglement in fishing gear, collisions with vessels, poaching and human disturbance.665 Of these, oil spills are the most significant risk.
Data gaps: There has not been a complete population count in B.C. since 1995. Little is known about the interchange between individuals in different populations and about habitat use. It would be helpful to clarify the importance of threats other than oil, such as disease, predation and entanglement in fishing gear.
D. Crustaceans
What are they? Crustaceans are a large group within the phylum Arthropoda (animals with external skeletons) and are second only to insects in their diversity of species.670 Barnacles, shrimps, crabs, amphipods (beach hoppers) and isopods (aquatic sow bugs) are among the types of crustaceans found in the intertidal zone. These animals are generally small to very small, with five or more pairs of legs. They can live within or on beaches or, as is the case for barnacles, attached to rocks in the intertidal zone. Crustacean life cycles vary, but most include a freeswimming larval stage that at times dominates the nearshore zooplankton community.
Why are they important? Intertidal crustaceans play two critical roles in coastal ecosystems.671 They recycle organic matter washed up on beaches and they provide a large, diverse prey base, which supports complex food webs that in turn promote ecosystem stability and resiliency.672 Intertidal crustaceans are decomposers, primary consumers or predators, and sometimes all three. Crustaceans, in general, occupy lower trophic levels. Species at lower trophic levels develop large populations, which ultimately support higher-level predators that are economically important to B.C. Amphipods and isopods are detritivores that recycle dead plant and animal material. An isopod commonly called the gribble is one of the few animals that can consume wood. Barnacles strain the planktonic 'soup' that flows over and past them, consuming a great variety of microscopic and slightly larger food items. Crabs are both decomposers and predators. Without functioning crustacean populations in the intertidal zone, the role of organic recycling becomes dominated by microscopic organisms. This becomes a cascading situation leading to anoxic (low oxygen) conditions, production of hydrogen sulphides, further degradation of environmental conditions and, as a result, the inability of the environment to support complex marine communities.673,674
Text Box 17. Ocean Acidification
Increased CO 2 in the atmosphere is having impacts beyond climate change. The ocean acts as a sink for CO 2, but this affects the acidity of seawater. 666 If global emissions continue to follow current trends, the average acidity could rise to a level higher than it has been for hundreds of thousands of years, representing a rate of change 100 times greater than at any time during this period. It could take tens of thousands of years to return to conditions similar to pre-industrial times.
Increasing seawater acidity gradually depletes the concentration of carbonate ions, which many sea creatures use to build shells and other types of exoskeletons. Recent research predicts that large parts of the ocean may lack this essential nutrient within a few decades and that by 2050 there could be too few carbonate ions in surface waters for shell formation.667 This trend is expected to continue and the effect will be most pronounced at higher latitudes.668
The effects on intertidal areas could be profound. Echinoderms (e.g., sea stars and sea urchins) have calcite structures containing magnesium, which dissolves very readily under more acidic conditions.669 These organisms often function as keystone species, grazing on algae and kelp. Mussels, oysters, copepods and crabs, which are all elements of intertidal ecosystems, are also affected.
Species at higher trophic levels that depend on crustacean productivity include salmon and shorebirds. Salmon smolts depend on a variety of crustacean prey, many of which are larvae of intertidal species. The timing of smolt migration coincides with major spawning and planktonic larval stages of crustaceans. Estuaries provide prey for grey whales (Eschrichtius robustus), including blue mud shrimp (Upogebia pugettensis), ghost shrimp (Callianassa californiensis) and larval intertidal crabs.675 Migrating shorebirds depend on feeding grounds along the coast of B.C., where they pause to feed on biofilm676 and a wide variety of crustaceans living on and in beaches.677
Status/threats in B.C. Generally, B.C. crustacean populations are stable, although declines in the diversity of species have been noted in some locations.678 Ocean acidification is a threat to all crustaceans (Text box 17).
Data gaps: There is no formal inventory for non-commercial species of crustaceans.
E. Seagrass Meadows
What are they? Seagrasses are flowering plants that have adapted to the marine environment.679 Seagrasses have similar structure to terrestrial grasses: rhizomal roots, long narrow leaves with obvious internal (vascular) structure and flowering stems. There are only a few species of closely related marine grasses in the genera Zostera (seagrasses, also known as eel-grasses) and Phyllospadix (surf-grasses). Along shorelines, where conditions are suitable, abundant growth of marine grasses forms ecosystems called seagrass meadows.
Why are they important? Seagrass meadows play an important role in primary production, carbon sequestration (processes that remove carbon from the atmosphere), habitat structure, shoreline stabilization and water quality maintenance.680 The productivity of a number of other important species is directly linked to healthy seagrass meadows. Pacific herring (Clupea pallasi), salmon and brant (Branta bernicla) are among the B.C. species that depend on seagrass meadows.
Status/threats in B.C. Seagrass meadows have been highly impacted by human activities. There are no accurate estimates of the pre-industrial range of seagrass meadows in B.C.,681 but Puget Sound, Washington, has lost an estimated 33% of its seagrass meadows since they were first inventoried.682 Extensive seagrass meadows develop in the same areas that humans find useful: estuaries and sandy shorelines with low wave action. Threats to seagrass meadows include log handling, vessel traffic, dredging, upland erosion and construction activities, shoreline structures, increased water temperature, pollutants, excessive nutrients, herbicides and invasive alien species.683
Data gaps: There are few science-based inventories of B.C. seagrass meadows. We do not know the ecological significance of specific populations and, in many cases, the reasons for declines are unknown.
F. Upland Sediments And Large Woody Debris In The Intertidal
What is it? Estuaries, tidal flats, salt marshes, sand dune complexes, beaches and sand spits receive sediments and large woody debris (LWD) from upstream areas, either directly from streams and rivers or indirectly via the ocean.684 Floods, storms and tides often later relocate these elements, contributing to the constantly changing nature of these ecosystems. In general, estuaries and coastal wetlands are net depositional environments for sediments.685
Sediments found in estuarine ecosystems are typically composed of animal and plant matter, as well as inorganic material such as mud or sand. Sources of large woody debris include woody vegetation from upstream riparian areas and drift logs that have broken free from log booms.
Why is it important? Estuarine ecosystems, both vegetated and unvegetated, are critical transition zones that link terrestrial, freshwater and marine habitats and perform many essential ecological functions, including nutrient cycling. Sediment-associated organisms - including bacteria, fungi, single-celled animals and sedimentdwelling invertebrates (e.g., nematodes, copepods, annelids, molluscs and peracarid crustaceans) and vascular plants - are integral to these functions. Sediment-associated plants also contribute to structural complexity in estuarine ecosystems, providing important habitat for a wide variety of species.686
Large woody debris plays numerous ecological roles in estuarine ecosystems. These include providing habitat and food for wood-degrading organisms such as wood-boring isopods (e.g., Limnoria lignorum) and shipworms (e.g., Bankia setacea), inputs into detrital food webs, shelter for fish from high current velocities and predators, egg attachment sites for fish such as Pacific herring, perches or nest sites for birds such as bald eagles, great blue herons, gulls (Larus spp.) and purple martins (Progne subis), haulout sites for harbour seals and colonization sites for woody vegetation. In estuarine marshes, LWD can have opposing influences on successional processes: stable pieces of wood act as nurse logs for trees and shrubs, while mobile pieces can keep the forest edge from advancing by battering against trees and the upper marsh shoreline.687,688
Status/threats in B.C. The main threats to estuarine ecosystems in relation to upland sediments and LWD are ecosystem conversion and degradation. Agriculture, urban development and the construction of port facilities and roads have all impacted estuarine ecosystems in parts of B.C., particularly around southern Vancouver Island and along the lower mainland coast.689 Human activities in inland areas have also affected these ecosystems.
Since the mid 1800s there has been a dramatic reduction in the volume and size of LWD in coastal ecosystems in the Pacific Northwest states;690,691 this trend likely also applies to B.C. The amount of LWD in estuarine ecosystems, especially large and long pieces of wood, has declined as riparian forests have been logged and as dams in some river systems have interrupted the downstream movement of LWD. Removal of logs and stumps to maintain channel navigability, diking, marsh filling and channelization have decreased the retention of LWD in estuarine ecosystems.692
Predicted impacts of climate change on estuarine ecosystems include erosion and/or sedimentation, coastal flooding and permanent inundation of low-gradient, intertidal areas. Unvegetated tidal flats are vulnerable to increased submergence and more extensive and rapid erosion. However, increased upland erosion and flooding associated with increased winter precipitation and more frequent winter storm and surge events, plus increased river flow due to glacial melt, may result in sufficient sedimentation to offset coastal erosion and submergence.693
There is evidence that estuarine marshes can adapt to sea-level rise provided there is sufficient sedimentation and internal biomass production and room for the entire wetland to move to higher ground or farther inland. If barriers to wetland migration are not removed, it is highly likely that climate change will result in significant shrinkage and eventual disappearance of salt marshes along the extensively diked Squamish, Nanaimo and Fraser rivers.694
Data gaps: There have been few scientific studies done to confirm or further characterize hypothesized ecological functions of estuarine LWD; this lack is in marked contrast to the extensive research on LWD in riverine and terrestrial ecosystems.695 There are also important gaps in knowledge of estuarine sediment-associated organisms and their functions. For example, we do not know whether rates of organic matter decomposition and nutrient recycling in estuaries depends on the diversity of species of microbes. We know more about the links between biodiversity and function for larger plants and animals, but many questions remain unanswered.696
G. Estuaries
What are they? An estuary is a partially enclosed body of water where sea water is measurably diluted by mixing with river runoff (see Section 2.2.3.2, p. 47).697 Estuaries can be classified as salt wedge (large river runoff with little mixing between the fresh water above and salt water below), partially mixed (greater tidal action and lower river runoff causing mixing, e.g., inlets, sounds) or well mixed (strong tidal action with low river runoff, resulting in water that is nearly homogeneous; typical of small bays near turbulent areas). Estuarine ecosystems have similar characteristics to wetland ecosystems, but also have daily fluctuations in the water table and variations in salinity.698
Estuaries are affected by several physical processes that are largely independent of human activities (e.g., tides, wind, rain, sunlight, evaporation, differences in water density). Human activities can affect estuaries by influencing the volume of water released by a river (e.g., through dams or water diversions), modifying the estuary opening through dredging, changing the runoff from surrounding land (e.g., by creating impervious areas) or releasing environmental contaminants.699
An estuary system can be subdivided into three areas: tidal river; zone of mixed fresh and salt water; and nearshore zone.700 As a result, an estuary includes the lower reaches of a river and the surrounding terrestrial land that is inundated infrequently (i.e., only at the highest tides), the intertidal zone (which is subject to daily tidal inundation) and the zone below the lowest tide (which is always covered by water).
B.C.'s two largest estuaries, those of the Fraser River and the Skeena River, have the second and fourth largest annual mean freshwater inflow from rivers, relative to all estuaries along the west coast of North America.701
Why are they important? Both organic and inorganic nutrients from rivers collect in estuaries to create biologically active areas where large populations of mammals, birds and marine organisms congregate,702 with primary production rivalling tropical rainforests.703 Estuaries are transition areas that provide connectivity for many aquatic migrating species, such as salmon, as they travel between the ocean and the upstream river. Estuaries fulfill ecological roles such as filtering water, decomposing organic matter and providing feeding habitat.704,705 Within estuaries, habitats such as seagrass meadows and wetlands are recognized as 'nurseries' (i.e., rearing areas), particularly for fish and invertebrates.706
Estuaries overlap with a number of other key elements in B.C., including riparian areas, stream systems, anadromous salmonids, waterfowl herbivory and crustaceans. Depending on geography, they may also contain macroalgae, seagrass meadows and willows. In B.C., hundreds of thousands of wintering waterfowl depend on estuaries.707 The Fraser River estuary supports the highest concentration of wintering birds (shorebirds, waterfowl and raptors) in Canada.708 Sometimes more than a million birds can be found in the Fraser River estuary on a single day and 20 million salmon pass through annually during a period of a few weeks.709
Status/threats in B.C. In the Georgia Basin, approximately 23% of the nearshore has been urbanized.710 The human population adjacent to the Fraser River estuary is similar in scale to that of other estuary sites along the west coast of North America, such as San Francisco Bay and Puget Sound. Key threats to west coast estuaries include ecosystem conversion and degradation, diverted fresh water flows, marine sediment contamination and alien species introductions.711 As a result, loss of estuarine habitat is substantial in the Fraser River delta (70%)712 and on the east coast of Vancouver Island (32%),713 with much of this area converted to fertile agricultural land through diking. Estuarine ecosystems are also threatened by sea-level rise resulting from climate change. Particularly vulnerable are those adjacent to dikes, which will prevent them from migrating to higher elevations.
With approximately 2.3% of B.C.'s rugged coastline classified as estuary, these ecosystems are considered rare.714 Estuaries in B.C. have been mapped at various regional scales (in 1984,715,716 1993717 and 2000718), as well as at a provincial scale (in 1985719 and 2007720). The 2007 estuary mapping defined intertidal polygons for over 440 estuaries in B.C., calculated the total intertidal area (75,000 ha) and ranked them for biological importance to water birds. In 1999, a large-scale mapping framework for describing the physical and biological character of estuaries was prepared for B.C.721
Data gaps: Intertidal areas of estuaries at the mouths of fourth-order rivers have not been mapped at a scale of 1:50,000 and the 1999 mapping framework for estuaries has not been applied to all B.C. estuaries. The condition of estuaries at regional and provincial scales is unknown. Knowledge of wildlife use of many estuaries is incomplete and knowledge of cumulative impacts on estuarine functions is limited.
2.5.2 Special Elements
B.C. has several elements of biodiversity that are of global significance either because they are important habitat for seasonal concentrations of species or because they are uncommon or even unique globally (Table 24).722 These elements are among the things that make B.C. special. They relate to geography, geology and the relatively undisturbed character of large areas of the province. Like many of the key elements described in Section 2.5.1, the special elements are subject to numerous threats that can potentially decrease the resilience of B.C.'s biodiversity. This list of special features is not intended to be all-inclusive, but rather to highlight some uncommon B.C. species, communities and ecosystems of ecological significance.
2.5.2.1 Seasonal Concentrations Of Species
A. Important Bird Areas
What are they? Eighty-four sites in B.C., many of which provide important habitat for concentrations of breeding, wintering and/or migrating birds, are recognized as Important Bird Areas (IBAs).723 These sites occur primarily along the coast, particularly around the lower mainland and Vancouver Island, and secondarily through the central interior and along the southern border with the U.S. (Map 11A). Examples of IBAs in B.C. include: the Scott Island Group off the northwest tip of Vancouver Island, which hosts 12 seabird species totalling more than two million breeding birds; Fraser Lake in central B.C., which is a globally significant site for wintering trumpeter swans; and the Skookumchuck Prairie, a small area of native grassland in the Rocky Mountain Trench that provides habitat for about 22 pairs of long-billed curlew (Numenius americanus), a species of conservation concern. One of the most important areas in B.C. for bird concentrations is the Fraser River estuary, which includes coastal wetlands, mudflats and intertidal marshes and provides habitat for a high diversity and biomass of birds.
Why are they important? B.C. provides important habitat for seasonal concentrations of many species of birds for breeding, wintering, and/or migrating, particularly along the Pacific Flyway (see also Section 2.3.4.3, p. 69). These sites are critical to key life stages for large portions of the total continental populations of these species.
Status/threats in B.C. Species occurring in localized high densities become vulnerable to outside threats. Most IBAs in B.C. are not protected. Current threats to the most significant IBA, the Fraser River estuary, include loss of intertidal habitat through planned industrial port expansion, intensification of agriculture (e.g., greenhouse development adjacent to the intertidal zone, berry and nursery crops) and pollution from the mouth of the Fraser River. Disturbance has grown in recent years (e.g., bird control at nearby Vancouver International Airport, increasing boat traffic, hikers and beach walkers), with potentially detrimental impacts on bird habitat and populations. Sea level rise resulting from climate change could reduce intertidal habitat.724 On some coastal islands, introduced mammals (e.g., mink [Neovison vison] and raccoon [Procyon lotor] on Cox and Lanz Islands in the Scott Island Group) are having detrimental impacts on ground-nesting seabirds.725
Data gaps: Many of these areas where birds congregate are surrounded by increasing development. We do not know the thresholds in terms of water quality and shoreline development for trumpeter swan populations that winter on Fraser Lake, or how much human disturbance populations on the coast can be subjected to without showing declines in reproductive success. Other unknowns are the impacts of fisheries on seabird colonies and how climate change will affect food resources.
B. Steller Sea Lion Rookeries And Haulouts
What are they? The Steller sea lion is the largest species of sea lion and the only one that lives year-round and breeds in B.C. waters. Steller sea lions prefer exposed rocky shorelines and wave-cut platforms for haulout sites and breeding areas (rookeries), returning to the same sites year after year.726 There are three main breeding areas in B.C.: the Scott Islands (including Beresford, Sartine and Triangle islands) off the northwest tip of Vancouver Island, the Kerouard Islands off the southern tip of Haida Gwaii/Queen Charlotte Islands, and North Danger rocks off the northern mainland coast (Map 11A).727
Why are they important? Two of B.C.'s rookeries are the largest breeding aggregations in the world.
Status/threats in B.C. Both breeding and non-breeding populations of the Steller sea lion are of conservation concern in B.C.728 Locations for haulouts and rookeries are mapped. Two of the three rookeries - those on the Kerouard Islands and the Scott Islands - are protected. Steller sea lions are vulnerable to shooting, incidental take in fishing gear, entanglement in debris, catastrophic events, environmental contaminants and displacement from, or degradation, of their habitat.
Thousands of Steller sea lions were culled, killed for research or killed for commercial reasons in the first half of the 20th century. This was very disruptive and caused the loss of at least one rookery. Populations have not fully recovered in most areas in spite of receiving full protection under the Fisheries Act in 1970. Currently, about 8,900 sea lions are found on rookeries in B.C., which represents about 65% of the number present prior to the large-scale kills. The total B.C. population inhabiting coastal waters during the breeding season is estimated at 18,400 to 19,700 individuals, including non-breeding animals associated with rookeries in southeast Alaska.729
Data gaps: More information is needed on rates of disturbance at haulout sites (e.g., by aircraft, boats, pedestrians, construction or fishing activities).730 The population-level impacts of oil spills are not well established.731
C. Major Salmon Spawning Sites
What are they? Native anadromous salmonids in B.C. include chinook, chum, coho, pink and sockeye salmon, and steelhead (see Section 2.5.1.3-F, p. 121). High levels of habitat diversity, variable environmental conditions and the strong tendency for salmon to return to their rivers of origin have combined to promote rapid, postglacial evolution of these species, resulting in many thousands of unique spawning populations specifically adapted to local conditions.732
Major salmon spawning sites (Map 11A) are located where spawning populations exceed a defined number of individuals in two or more years (i.e., >20,000 pink salmon, >10,000 chum salmon, >5,000 lake-type sockeye salmon, >2,000 coho salmon, >500 chinook salmon, >500 river-type sockeye salmon).733,734
Why are they important? A disproportionate number of B.C. salmon come from major spawning sites. These populations exhibit considerable genetic diversity below the species level, reflecting the evolution of local adaptations that especially suit them to occupy a given geographic locale.735 In addition to being important to biodiversity, major salmon spawning sites are culturally significant to First Nations (see Text box 2, p. 13).
Status/threats in B.C. The number of major spawning sites for each species is: 189 for chum salmon, 183 for coho salmon, 89 for even-year runs of pink salmon, 65 for odd-year runs of pink salmon, 125 for both oddand even-year runs of pink salmon (i.e., sites where both thresholds are met), 55 for chinook salmon and 46 for sockeye salmon.736
The greatest threats to major salmon spawning sites are climate change and conversion or degradation of spawning and rearing habitat (see Section 2.5.1.3-F, p. 121). The seasonal concentration of salmon populations during certain parts of their life cycle contributes to their vulnerability to catastrophic events. For example, on August 5, 2005, a train derailment resulted in a spill of approximately 45,000 L of sodium hydroxide solution into the Cheakamus River. Nearly all free-swimming fish occupying the Cheakamus River mainstem were killed, with mortality estimated at more than 500,000 fish from 10 species. The only survivors were those fish that were still in the gravel as developing young or were residing in tributary streams or back channels, and those that had not yet returned to the Cheakamus River.737
Data gaps: Major salmon populations and spawning sites are well studied. The effects of climate change on the future use of these sites are not known.
2.5.2.2 Special Communities
A. Old-Growth Temperate Rainforests
What are they? Temperate rainforests occur in mid latitudes and are typically associated with the ocean and coastal mountain ranges, which promote high rainfall. B.C. has coastal rainforest along its entire coast and on its offshore islands, as well as inland rainforest located between 51°N and 54°N along the windward slopes of the Columbia and Rocky mountains.738 Another definition of the inland temperate rainforest gives a larger range, extending from south of the U.S. border to Prince George.739 In B.C., the coastal temperate rainforest is largely described by the Coastal Western Hemlock and Mountain Hemlock biogeoclimatic zones and the inland temperate rainforest by the Interior Cedar-Hemlock zone. Old-growth temperate rainforest is defined as more than 250 years old (Map 11B).
Temperate rainforests are primarily dominated by a disturbance regime called gap dynamics, meaning that, in general, single trees die, creating gaps, and are replaced by trees growing up from the understory, rather than entire stands being replaced at once. This results in forests that are very old and complex, with multiple layers of trees. Forest stands are often older than the individual trees within them and may not have been disturbed for many thousands of years. Such stands have been called 'antique' forests.740 On the west coast of Vancouver Island, stands have been aged as having existed for more than 3,000 years since the last disturbance.741,742 In B.C.'s inland temperate rainforest, researchers have found trees greater than 1,400 years old and stands that have survived through multiple generations of trees.743
Some temperate rainforest areas are influenced by disturbances such as large-scale windthrow (e.g., northern Vancouver Island and exposed parts of the outer coast), large-scale fire (e.g., the south coast and the drier zones of the Interior Cedar-Hemlock zone) and flooding (e.g., riparian areas). The coastal rainforest grows some of the world's largest trees, with some Sitka spruce exceeding 90 m in height and western redcedars reaching more than 15 m in circumference.744 Although inland rainforest trees typically do not achieve these dimensions, they are regionally exceptional for their size. In contrast to the largestructured forest, parts of the coastal temperate rainforest consists of woodland bog forest, characterized by widely spaced, small, stunted trees and little woody debris or other structure. Riparian forests are also prevalent in both coastal and inland temperate rainforests.
Why are they important? B.C.'s old-growth rainforests contain high biodiversity. Their large structures (i.e., standing trees and coarse woody debris) and multiple layers provide habitat niches for many species. This complexity combined with the great age of many of these forests affords time and opportunity for speciation. The high diversity of non-vertebrate species inhabiting these forests is particularly notable. For example, in coastal rainforests, more than 300 species of previously unknown arthropods have been identified in the Sitka spruce canopy, none of which have been found in young regenerating forests,745 and 83 species of oribatid mites have been found in the canopy and in areas of the forest floor associated with western redcedars.746 Both coastal and inland old-growth rainforests support a high diversity of lichens and fungi.747,748
Bog forest ecosystems within the coastal rainforest are typically nutrient-poor, which tends to result in relatively high plant diversity due to the inability of single species to dominate these sites.
There are 242 species of provincial conservation concern in the Coastal Western Hemlock zone, 45 in the Mountain Hemlock zone and 170 in the Interior Cedar-Hemlock zone (see Table 4, p. 34). Some of these species occur in more than one of these zones.
Status/threats in B.C. Researchers estimate that 56% of the world's coastal temperate rainforest has been logged or converted to non-forest uses. In North America, the only remaining large, unlogged watersheds are in British Columbia (mainly on the central and north coasts) and Alaska.749 The world's largest area of intact coastal temperate rainforest (321,120 ha) is encompassed by the Kitlope Heritage Conservancy Protected Area on B.C.'s mainland coast.750
Much of B.C.'s remaining old-growth coastal temperate rainforest is fragmented by roads, other access routes and forest harvesting. Almost half (47%) is in small, unfragmented areas that are less than 20,000 ha in size. Approximately one-third is in unfragmented areas of greater than 50,000 ha.751
Development has been concentrated in low-lying, high-productivity areas and very little old forest remains in these sites.752,753,754 Depending on how the inland temperate rainforest is defined, either all or most of this ecosystem occurs in B.C. All of the province's old-growth inland rainforest is highly vulnerable to the impacts of roads, logging and flooding by hydroelectric dams. Much of this ecosystem has already disappeared and the remaining stands are highly fragmented.755
Data gaps: The mapping of old-growth temperate rainforests in B.C. is out of date and incomplete for some areas. The ecology of these forests is not well understood. Recovery times for different rainforest elements and the overall recovery ability of these forests are unknown, particularly in the context of climate change.
B. Intact Large Mammal Predator-Prey Systems
What are they? British Columbia is home to four species of large carnivores (i.e., with body mass greater than 20 kg): grey wolf, cougar, American black bear and grizzly bear. Large herbivores historically or currently preyed on by this suite of predators include all of the province's native ungulate species: plains and wood bison (Bos bison bison and B.b. athabascae), mountain goat, bighorn and thinhorn sheep (Ovis canadensis and O. dalli), moose, elk, mule and white-tailed deer (Odocoileus hemionus and O. virginianus) and caribou.
An intact large mammal predator-prey system is one in which all of the native species are present, with no alien species that plays a role as either predator or prey relative to the others. These systems are vital elements in many natural communities (see Section 2.5.1.2-C, p. 100).
The loss of one or more species from a large mammal predator-prey system can result in a variety of ecosystem impacts. The absence of top predators can lead to an artificially high abundance of herbivores and smaller, generalist predators, whose influence can ripple through the ecosystem, producing impacts such as overgrazing, overbrowsing, declines in populations of ground-nesting birds and spread of alien species.756
Why are they important? B.C. is globally significant for the fact that 28% of the province has intact large mammal predator-prey ecosystems and these systems are unusually rich in temperate-zone, large carnivore and ungulate species. Many of these species have undergone significant range contractions in other jurisdictions, which increases the significance of their presence in B.C. Worldwide, less than 21% of the earth's land base retains all of the large mammal species that were historically found in each area.757
The presence or absence of intact large mammal assemblages, including the full historical complement of large carnivores and ungulates, is a useful ecologically based measurement of human impacts on biodiversity. In general, areas with intact large mammal assemblages are more likely to be ecologically functional than those that are missing one or more species.758
Status/threats in B.C. Large mammals are particularly vulnerable to local extirpation due to direct mortality (depending on the species, they may be killed for meat or trophies or as a population-control or protective measure) and the sensitivity of some of these species to disturbance and habitat fragmentation.759 A continentwide analysis of sensitive species (i.e., those that have undergone significant range contractions) of carnivores and ungulates in North America shows B.C. to be one of the areas with the highest number of these species, both historically and currently. Species richness for sensitive carnivores and ungulates is particularly high in the northern Rocky Mountains from Colorado to the Yukon, but is also high in parts of B.C. outside the Rockies (Figure 30).760 B.C.'s large mammal fauna is intact along the mainland coast, but there are gaps elsewhere in the coastal region (see Map 11A, p.138). Specifically, Dawson caribou, known only from Haida Gwaii/Queen Charlotte Islands, are extinct, and Roosevelt elk (Cervus canadensis roosevelti) have been extirpated from parts of Vancouver Island, the Sunshine Coast and the lower mainland.
In most of the rest of B.C., one or more large mammal species have been extirpated since the time of European contact. Wood bison are missing from most of northern B.C. and grizzlies from much of the central and southern interior. The other species most frequently missing from predator-prey systems in areas of B.C. are wolves, plains bison, elk and caribou. The primary reason for these losses is historical unregulated direct killing.761
Attempts to reintroduce extirpated large mammals into their former range have been made in B.C. Experience elsewhere has proven that such reintroductions can have dramatic, positive ecological effects.762
Data gaps: The mapping of historic and current distributions of some large carnivores and ungulates is incomplete (e.g., historic distribution of wood bison, current distribution of grey wolf).
2.5.2.3 Special Features
A. Large Wetlands (Freshwater)
In B.C., wetlands tend to be small, isolated features. Three exceptions in the province are: Burns Bog, the largest raised bog in coastal North and South America; and two internationally recognized wetland complexes in the Kootenays - the Creston Valley Wildlife Management Area and the Columbia Valley Wetlands Wildlife Management Area (see Map 11B, p. 142).
Creston Valley Wetlands
What is it? The Creston Valley wetland complex is a large area (approximately 7,000 ha) at the south end of Kootenay Lake. It consists of Duck Lake (1,500 ha), 17 marshes and a portion of the Kootenay River.
Why is it important? The area provides habitat to more than 265 bird species, 50 mammal species and 30 fish species, as well as reptiles, amphibians and thousands of invertebrate and plant species. It is an important stopover site for tundra swans (Cygnus columbianus), greater white-fronted geese (Anser albifrons) and many other waterfowl. It is also a regionally important site for birds of prey wintering in the B.C. interior and home to the northern leopard frog (Rana pipiens), a species of conservation concern. The marshes are a valuable link in a chain of wetlands stretching from the Arctic Ocean to California.
Figure 30: Historic (a) and current (b) species richness for 17 carnivore and ungulate species that have undergone significant range contractions in North America.
Source: Laliberte, A.S. and W.J. Ripple. 2004. Range contractions of North American carnivores and ungulates. Bioscience 54(2): 123-138.
Status/threats in B.C. Most of the complex is managed under the Creston Valley Wildlife Management Area Act. Threats to the Creston Valley wetlands include continued ecosystem conversion, runoff from surrounding agricultural and urban areas and the requirement for continuous management to maintain the current artificial water levels that maintain wetland productivity. The wetland complex is also intersected by highway and railway corridors.
Columbia Valley Wetlands
What is it? The Columbia Valley wetland complex in the East Kootenay Trench is the longest contiguous network of wetlands in North America, covering more than 26,000 ha.
Why is it important? These wetlands host more than 260 resident and migratory bird species, including some that are rare within the province (e.g., prairie falcon [Falco mexicanus], short-eared owl [Asio flammeus] and American avocet [Recurvirostra americana]).
Status/threats in B.C. Much of this complex is managed within several Wildlife Management Areas. Threats to the Columbia Valley wetlands include runoff from pesticides and fertilizers used in surrounding areas, which affects wetland function, and increasing recreational impacts. Motorized recreation using power boats and jet skis is of particular concern because it can result in erosion and destruction of nesting habitat through increased wave action, and can also cause nest desertion.
Burns Bog763
What is it? Burns Bog, in the Fraser River delta, is a type of wetland known as a raised bog, which typically develops on top of a fen. In a raised bog, the supply of mineral-rich groundwater is cut off as the bog rises, leaving nutrient-poor precipitation as the bog's only source of moisture and nutrients. Raised bogs are characterized by acidic, nutrient-poor water, two-layered peat deposits and communities dominated by plants that can survive in these conditions, such as Sphagnum and heaths (Ericaceae).764
Why is it important? Although relatively common in the North America's boreal and eastern regions, raised bogs are less common in western North America, where blanket bogs or fens predominate. Burns Bog is the largest raised bog on the west coast of the Americas, covering 4,000 ha, and is at the southern limit of some northern species.765 Raised bogs host uncommon collections of species, including unusual numbers of carnivorous plants, an apparent adaptation to lack of nutrients. Plant species in Burns Bog that are uncommon to the region include the carnivorous great sundew (Drosera anglica), as well as few-flowered sedge (Carex pauciflora) and crowberry (Empetrum nigrum). The bog is also home to animals of provincial conservation concern, including sandhill cranes and the occidentalis subspecies of the southern red-backed vole. A new species, the Olympic shrew, was recently confirmed from Burns Bog.766
Like other peatlands, raised bogs sequester greenhouse gases (both methane and CO 2), thus acting as a buffer against climate change. Disturbance of the bog surface increases emission of these gases.
Status/threats in B.C. About half of this wetland (2,042 ha) is protected as the Burns Bog Ecological Conservancy Area, but it is threatened by incursion of 'non-bog' water that is not favourable to the bog flora and fauna. Much of the remainder of the bog is privately owned and is occupied by a large landfill.
Large wetlands data gaps: Burns Bog and the two Kootenays wetland complexes are well mapped. However, the impacts of land uses in surrounding areas are not well known. The ecological and hydrological processes of Burns Bog, and bogs in general, are not well understood,767 particularly the requirements for succession and recovery.768
B. Karst
What is it? Karst is a landscape derived from soluble bedrock - typically limestone, but also dolomite, marble and gypsum. With time, water dissolves the bedrock and creates caves, sinkholes, vertical shafts, convoluted rock surfaces and disappearing and reappearing streams. Approximately 11% of British Columbia is potentially underlain by karst, including the Rocky Mountains (particularly in the south) and the temperate rainforests of Vancouver Island and the Queen Charlotte Islands; there are also smaller, more isolated patches on the north coast, in the Purcell Mountains, in the northwest along the Stikine and Taku rivers, in the northeast near Chetwynd and Prince George, and near Chilliwack in the Cariboo.769,770 The province appears to have the most diverse examples of karst in Canada.771
Why is it important? Some habitats formed from karst are naturally isolated and therefore tend to host rare or unique species. Karst caves in B.C. are inhabited by species found nowhere else and are important as bat hibernacula.772 Above ground, the complex physical structures formed in karst stream beds provide sites for fish to rest, breed and avoid predation. Terrestrial ecosystems on karst also tend to be very productive because of high levels of dissolved minerals, fractured bedrock and well-drained soils, all of which encourage deep rooting and high growth rates of associated plants and trees. Their chemistry is basic rather than acidic, allowing colonization by plant species (including bryophytes, ferns and rooted plants) that are rare or absent on more acidic substrates such as granite. This chemistry, plus their productivity, encourages high species richness. Karst sites in B.C. are recognized for their rare plant species and cave fauna, including some endemic species.773,774
For example, caves on Vancouver Island host at least 10 species of invertebrates found nowhere else and others that are extremely rare.775
In Alaska, karst landscapes have been shown to increase the productivity of adjacent salmon streams by producing cool, stable streams with ideal properties for fish spawning 776 and by leaching of calcium carbonate, which buffers acidic streams; aquatic insect populations tend to be more diverse within karst-fed streams. Beyond their contributions to biodiversity, karst ecosystems are often of cultural value to First Nations, including as burial sites,777 and are important repositories for historical evidence of climate change (sediments and formations) and extinct species.778,779
Status/threats in B.C. Karst ecosystems in B.C. are highly vulnerable to disturbance particularly those found in the wet, mountainous coastal regions of the province. Karst located in the interior is generally less susceptible to surface development due to the protection provided by deep soils and surficial materials deposited through glaciation. Road building and logging can cause direct physical damage, soil erosion and sediment transfer, and can interrupt natural surface and subsurface drainage patterns and collapse caves, potentially causing the collapse of the entire karst ecosystem.780
Because karst is highly productive, associated forests are sought after for timber harvesting. However, the geological characteristics that encourage productivity also encourage soil loss and site degradation once vegetation cover is disturbed. Indirect damage, such as sedimentation from fine-textured soils and blockages caused by debris, can also affect the functioning of karst ecosystems. Just over half of the B.C. landscape that is potentially karst is forested and, of that, 55% has been logged.781
In some areas there is high recreational use by cavers.
Data gaps: Provincial mapping of karst sites is incomplete and relatively little assessment of karst-associated species has been done.782 It is also unlikely that all noteworthy sites are known.
C. Hot Springs
What are they? Hot springs are habitats created by pools of very hot, sometimes near boiling, water that is heated from deep within the earth. Hot springs occur on every continent. Within Canada, they occur in the mountainous regions of B.C. (see Map 11B, p. 142), Alberta, the Northwest Territories, Yukon and Nunavut.
Why are they important? Because of the uncommon conditions in hot springs (very high temperatures, little or no oxygen and large amounts of dissolved minerals), hot springs habitat is very different from surrounding habitats. Hot springs are highly localized and isolated from each other and tend to host very simple but unique ecosystems and species. Only one species in B.C. is reported to be restricted to hot springs: the hotwater physa (Physella wrighti) is a critically imperilled freshwater snail that occurs only in the Liard Hot Springs783 (i.e., it is endemic to B.C. and to that particular site). In B.C., the southern maiden hair fern (Adiantum capillus-veneris) is reported only from the Fairmont Hot Springs but also occurs on five continents and is sufficiently secure in some areas that it is harvested by florists. Other plants sometimes occurring near hot springs in B.C. are the giant helleborine (Epipactis gigantea) and marsh muhly (Muhlenbergia glomerata), which have limited distributions in the province. Both are relatively widespread in North America, but giant helleborine is localized and often threatened. There undoubtedly are smaller organisms, such as microbes and possibly invertebrates, restricted to B.C. hot springs.
Status/threats in B.C. Locations of hot springs within British Columbia are known. Most larger and accessible ones are privately owned and developed. Several occur within provincial parks. Among the best known are Liard River Hot Springs Provincial Park, Ahousat Hot Springs (Gibson Marine Provincial Park) and Hot Springs Cove (Maquinna Provincial Marine Park). All attract visitors. Their small size, isolation and attractiveness to humans make hot springs especially vulnerable to human activities.
Data gaps: Most hot springs have not been surveyed for their contributions to biodiversity, particularly for smaller organisms.
D. Glacially Influenced Streams
What are they? Glaciers cover approximately 4% of B.C.'s land area (see Figure 7, p. 25). They serve as frozen reservoirs of water that feed lakes and rivers during the late summer and fall when runoff from seasonal snow cover is depleted. Almost half of the gauged rivers in the province have at least one glacier in their basin.784 Glacially influenced watersheds are defined here as those with more than 5% of their area covered by glaciers, and streams within these watersheds are considered to be glacially influenced.785 Glacially influenced watersheds cover 20% of the province (Map 11B, p. 142). In glacier-fed rivers, the highest flows tend to occur in early or mid summer, depending on latitude, and glacier runoff can account for a significant portion of the available water supply.786 Daily flow patterns are also distinctive. Near its source, the volume of a glacial river can have as much variation during a single summer day as over the course of an entire summer. Glacial rivers run high in the late afternoon and early evening in summer, with peak water levels on hot summer days sometimes reaching as high as during spring thaw. After sunset, melting slows, and by morning, water flow is greatly reduced.787
Why are they important? In late summer and fall, glacier melt maintains stream flow during dry weather, providing a reliable water supply and valuable aquatic habitat in downstream waterways and associated riparian and estuarine ecosystems. Glaciers can also affect stream temperature and may be important to cold-water specialists such as bull trout.
Status/threats in B.C. Glaciers in B.C. are generally shrinking due to climate change and glacially influenced streams are threatened by the resulting changes in glacier melt. Initially, receding glaciers discharge more water into some streams and rivers. While higher flows may benefit some aquatic species, potential negative impacts include increased stream turbidity and damage to fish habitat and riparian areas. In the longer term, glacier retreat will mean reduced water volume, and possibly temperature change, in glacier-fed streams and rivers, especially during the summer months.788
A recent study of August stream flow in 236 basins in B.C. showed significantly more negative trends for glacier-fed than for non-glacier-fed streams, supporting the hypothesis that retreating glaciers have exacerbated recent summer low flows. The negative stream flow trends in glacier-fed catchments suggest that summer stream flow in B.C. will decline if current climate change trends continue and glaciers continue to recede.789
Data gaps: There is no complete listing of species that are dependent on glacially influenced streams in B.C. There is a lack of stream monitoring data to gauge changes in the rate of water flow and temperature.
E. Serpentine Soils
What are they? Serpentine soils are derived from serpentine and other rocks typical of the earth's mantle. These rocks contain high levels of magnesium, chromium, manganese, cobalt and nickel, which make the soils toxic to many plants. Serpentine soils also are characterized by a low calcium/magnesium ratio and low levels of potassium and phosphorus - minerals important for plant growth. The resulting vegetation is sometimes termed 'serpentine barrens.' Forests are absent or stunted, shrubs are usually sparse, and species adapted to serpentine soils are typically slow-growing.790 Serpentine soils occur worldwide in areas of tectonic plate activity, but occupy less than 1% of the earth's surface, so are not common anywhere. In B.C., serpentine soils follow a line of tectonic activity through the centre of the province from Tulameen Lake in the south to Atlin in the northwest.
Why are they important? Plants that can tolerate the harsh environment of serpentine soils often cannot compete successfully with other species in less hostile environments, so are restricted to serpentine ecosystems. Examples in B.C. include Lemmon's holly fern (Polystichum lemmonii) found on Mount Baldy in the Okanagan and the mountain holly fern (P. scopulinum) from the Tulameen River valley. Both species are relatively widespread (though localized) and considered globally secure, but are rare in B.C. The presence of serpentine ecosystems increases the overall biodiversity in B.C. by providing a niche for these specialized plants.
Status/threats in B.C. The overall status of serpentine soils is unknown. Because of their lack of productivity there is a tendency to treat areas of serpentine soils as wasteland. In B.C., the major threat is likely from mineral exploration and development, and possibly urbanization. Elsewhere, efforts to make serpentine ecosystems productive have involved massive intrusions that eliminate species adapted to these ecosystems.
Data gaps: No systematic, province-wide survey of these ecosystems has been undertaken.
F. Saline Lakes
What are they? Saline or 'salt' lakes have been defined as those having more than 3g/L salt content, in contrast with 'freshwater' lakes, which have less than 3g/L of salt. The 'salts' include sodium, magnesium, carbonate, bicarbonate and sulphate. Salt lakes or ponds have no outlet. They are formed where evaporation exceeds rainfall and minerals become concentrated by evaporation during the summer. They often are alkaline, have extremely high nutrient levels, trace metals and low oxygen. Often by the end of summer, saline lakes are surrounded by crystalline salts, and some areas dry up completely, resulting in small salt flats. Maritime glasswort (Salicornia maritima) may add a red margin around some ponds, while alkali saltgrass (Distichlis spicata var. stricta) adds a yellow tint to some crystalline rings. The lakes typically have few or no submergent plants and few emergents.
Saline lakes occur on all continents and are widespread in dry environments. B.C.'s southern Interior Plateau, particularly in the Okanagan and Kamloops areas and throughout the Fraser Plateau, is dotted with small saline ponds that are mostly less than 1 km2 in area (see Map 11B, p. 142).
Why are they important? The unique hydrology and species composition of saline lakes separates them from the surrounding habitat. Some species, including some dragonflies and midges (chironomids) and other invertebrates, such as brine shrimp (Artemia spp.), are specifically adapted to these conditions. For species that can maintain their internal chemical balance under these conditions, the lakes are secure and free of most potential predators. Adapted invertebrates can thus attain large numbers, resulting in ideal stopover and nesting sites for birds such as phalaropes (Phalaropus spp.) and plovers (Charadrius spp.). A very few saline lakes, such as Mahoney Lake in the Okanagan, are meromictic, meaning the water layers do not turn over, so there is no mixing. In Mahoney Lake, this lack of mixing permits the densest population of phototrophic sulphur bacteria (dominated by the purple sulphur bacterium [Amoebobacter purpureus]) ever reported in a natural body of water. The sulphur bacteria form a thin but highly concentrated layer, called a sulphur bacterial plate, at the boundary between the layers of water with and without oxygen. This bacterial plate, in turn, supports a copepod (Diaptomus connexus) and a rotifer (Brachionus plicatilis).791 Some saline lakes, such as Spotted Lake in the Okanagan, are culturally significant to First Nations. The lake and surrounding land has been purchased by the Okanagan Nation Alliance for the purpose of conservation.
Status/threats in B.C. Some saline lakes, such as Mahoney Lake and Soap Lake, are protected within ecological reserves. Saline lakes are especially vulnerable because they occur in dry areas where agricultural and rural development are prevalent. Climate change poses a particular threat to saline lakes because they are typically shallow and distant from taller vegetation and consequently not buffered from air temperature change. Although the lakes are valuable to some species, it appears that little can be done to sustain many of them in the face of climate change. Pollution and the introduction of exotic species are also threats. In some areas, salt lakes are an economically important source of minerals such as halite, uranium, zeolites and borax.
Data gaps: Listing and mapping of saline lakes within the province is incomplete. Their small size means they are usually overlooked in broad-scale inventory.
G. Fishless Lakes
What are they? Fishless lake is a term applied to freshwater lakes that, because of their physical isolation from other water bodies, do not contain fish and have not been stocked with fish. They occur throughout B.C., but are particularly common in mountainous regions where access by downstream fish is prevented by waterfalls or canyons, and on plateaus where isolated lakes have no inflow or outflow.
Why are they important? Although historically common in B.C., fishless lakes are becoming increasingly rare in the province and elsewhere, and are a special element because they provide a unique environment. Prior to stocking, approximately 95% of an estimated 16,000 mountain lakes in the western United States were fishless. Now 60% of all of these lakes and 95% of the deeper (more than 3 m) and larger (more than 2 ha) lakes contain introduced trout.792 Much the same has happened to accessible lakes at lower elevations in British Columbia.793 Fishless lakes and headwaters are special in that resident species are not subject to predatory fish. The community composition in fishless lakes is therefore different from that found in lakes with fish, and includes species such as rare cladoceran crustaceans and the tiger salamander (Ambystoma tigrinum), a species of conservation concern. These communities may not be able to effectively withstand predation.
Status/threats in B.C. In much of the province, fewer than 5% of the low-elevation lakes remain natural,794 and, even where habitats are protected, diversity may not be conserved.795 Long-term studies on the effects of introduced fish show that even when the introduced fish populations are extirpated, their impacts on community structure may continue up to a decade after the last fish introduction. In other lakes, the fish successfully reproduce and become a permanent predator, even spreading to other previously fishless lakes in a watershed. The practice of introducing game fish into fishless lakes has effectively eliminated the fishless lake ecosystem from large areas of western North America.
Data gaps: There is no consolidated list or mapping of fishless lakes.
H. Microbialites
What are they? Microbialites are fossilized mats formed by microbes, primarily cyanobacteria (previously known as blue-green algae).796 Cyanobacteria create microbialites by trapping sedimentary grains, binding the grains with mucous, and cementing them with lime into thin layers. The bacteria extend the microbialites vertically to access sunlight necessary for photosynthesis. The resulting structures have a variety of shapes: columnar, conical, branching or stratiform. Over millennia, these structures became fossilized limestone, and microbialites today have a very definite laminated structure and appearance. Many examples date from the Precambrian era, but some microbialites are still home to living microbes. Two examples are found in B.C. in Pavilion Lake and Kelly Lake (see Map 11A, p. 138).
Why are they important? Cyanobacteria are among the earliest living organisms on the planet and likely the first to photosynthesize, using water, sunlight and carbon dioxide to yield oxygen and calcium dioxide (lime). Fossilized bacteria sometimes found in microbialites are evidence of some of the earliest life forms, dating from approximately 3.5 billion years ago, and may provide clues to the origins of life on earth.797
Status/threats in B.C. Microbialites are rare and vulnerable to disturbance due to their fragility. One of the two known occurrences of microbialites in B.C. is found in Pavilion Lake in Marble Canyon Provincial Park. The Pavilion Lake microbialites, which reach 2 m in height, are the largest known freshwater microbialites and contain both cyanobacteria and diatoms. In nearby Kelly Lake, the microbialites were thought to be only 1-2 cm in height.798 Recently, however, much larger (>50 cm) structures have been measured.799 Kelly Lake is adjacent to Downing Provincial Park, but is not itself protected.
Data gaps: Although all microbialite sites may have been found, extensive surveys in candidate lakes have not been undertaken.



 Copyright 2007 Biodiversity BC
Copyright 2007 Biodiversity BC